��Ŀ����
��10Ϋ��24����(10��)̼������(NaHCO3)�׳�С�մ���һ�ְ�ɫ���壬�DZ��Ƹ�����õķ��ͷ۵���Ҫ�ɷ�֮һ��������ϡ������ᷴӦ����CO2���Իش�
(1) д��NaHCO3��ϡ���ᷴӦ�Ļ�ѧ����ʽ ��
(2)�����98��������(�ܶ�Ϊ1��849��mL)����980918��4����������Һ?
��
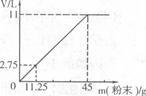
(3)�ֽ�45g NaHCO3(����KHCO3)�����ĩ����100mLϡ���ᣬǡ����ȫ��Ӧ��ʹ����ȫ���ݳ��������ĩ�����������CO2����Ĺ�ϵ��ͼ(��״���£�CO2���ܶ�Ϊ2g��L)��ͨ�����㣺
����100mLϡ�����������������
����ϡ����Ϊ120mLʱ����������ĩΪ58.5g�������CO2�������

(1) д��NaHCO3��ϡ���ᷴӦ�Ļ�ѧ����ʽ ��
(2)�����98��������(�ܶ�Ϊ1��849��mL)����980918��4����������Һ?
��
(3)�ֽ�45g NaHCO3(����KHCO3)�����ĩ����100mLϡ���ᣬǡ����ȫ��Ӧ��ʹ����ȫ���ݳ��������ĩ�����������CO2����Ĺ�ϵ��ͼ(��״���£�CO2���ܶ�Ϊ2g��L)��ͨ�����㣺
����100mLϡ�����������������
����ϡ����Ϊ120mLʱ����������ĩΪ58.5g�������CO2�������
(10��)
(1)2NaHCO3+H2SO4=Na2SO4+2CO2��+2H2O(2��)
(2)��100mL98����H2SO4�����ձ��ڱ���������796mLˮ�У�ͬʱ�ò��������Ͻ��衣(�÷�Ҫ�㣺100mLŨ�����1�֣�796mLˮ��1�֣�ŨH2SO4����ˮ�е�1�֣���3��)
(3)�⣺��m(CO2) ==11L��2g��L="22" g(1��)
��������Һ��H2SO4������Ϊx��
��(1)ʽ�ã�H2SO4 ~ 2CO2
98 88
x 22g
x=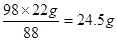 (2��)
(2��)
����120mLϡH2SO4��ȫ��Ӧ�Ĺ����ĩ������Ϊy��
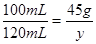 y=54g<58.5g
y=54g<58.5g
�����ĩ������������������м��㣺
V(CO2)== (2��)
(2��)
��(��)
(1)2NaHCO3+H2SO4=Na2SO4+2CO2��+2H2O(2��)
(2)��100mL98����H2SO4�����ձ��ڱ���������796mLˮ�У�ͬʱ�ò��������Ͻ��衣(�÷�Ҫ�㣺100mLŨ�����1�֣�796mLˮ��1�֣�ŨH2SO4����ˮ�е�1�֣���3��)
(3)�⣺��m(CO2) ==11L��2g��L="22" g(1��)
��������Һ��H2SO4������Ϊx��
��(1)ʽ�ã�H2SO4 ~ 2CO2
98 88
x 22g
x=
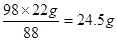 (2��)
(2��)����120mLϡH2SO4��ȫ��Ӧ�Ĺ����ĩ������Ϊy��
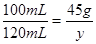 y=54g<58.5g
y=54g<58.5g�����ĩ������������������м��㣺
V(CO2)==
 (2��)
(2��)��(��)
��

��ϰ��ϵ�д�
�����Ŀ
 3Fe + 4CO2�����������ս������С�����һλ��
3Fe + 4CO2�����������ս������С�����һλ��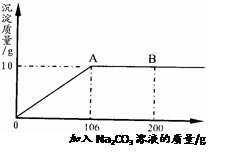
 CH4 + 2H2O��������66 g������̼����ת�����õ�����������Ƕ��١�
CH4 + 2H2O��������66 g������̼����ת�����õ�����������Ƕ��١�