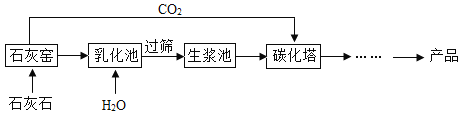
��Ŀ����
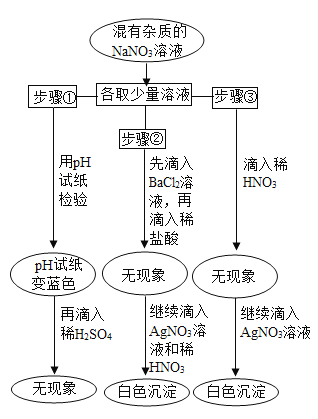
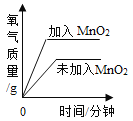
����Ŀ��ijѧ��ѧϰ��˫��ˮ��������ʵ��������Լ�����Ҳ��һƿ������˫��ˮ�����ڱ�ǩ�Ѳ����������ж����Ƿ���ʣ�����������ƿ��Һ����ѧУ������������ͼ��ʾ���о����Իش�
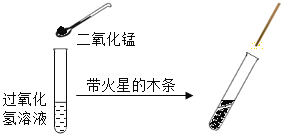
��1������������̵�������___________
��2���������Һδ���ʣ�Ӧ�ù۲쵽��������_______�������ܹ۲쵽��������˵����ƿ˫��ˮ�Ѿ����ʳ�_________��
��3����δ���ڵ�����˫��ˮ�����˿��ϣ���Ѹ�ٳ��ִ������ݣ�����Ϊ�����������_______
���𰸡������ã���ӿ췴Ӧ�ٶȣ� �����ݲ��� ˮ ����
��������
��1�����������ֽܷ�����ˮ�����������ʣ�����������������ܼӿ췴Ӧ���ٶȣ����Ա����Ϊ�������ã���ӿ췴Ӧ�ٶȣ���
��2�������Һδ�������ֽܷ����������ð�����ݣ������ܹ۲쵽���ݣ�˵����������ֽ������ˮ�����Ա����Ϊ�������ݲ�����ˮ��
��3����������ֽ�����������ˮ���ʴ�Ϊ��������

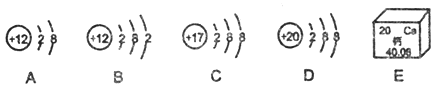
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�����Ŀ��ʵ������һƿ��ǩ��ȱ����ƿ��û����ȫ�ܷ����ɫ��Һ����ѧС���ͬѧ����ѯ����ʦ��ֻ֪����NaCl��NaOH��Na2CO3��NaHCO3�е�һ����Һ��Ϊȷ��ԭƿ���Ǻ������ʲ��ж����Ƿ���ʣ���ѧС���ͬѧ���������µ�̽�����
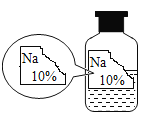
�����в��룩
��������ԭ��Һ��������NaCl�� ��������ԭ��Һ��������NaOH��
��������ԭ��Һ��������Na2CO3�� ��������ԭ��Һ��������NaHCO3��
��ǩ�����ȱ��ԭ����________��
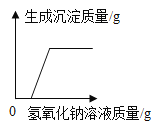
���������ϣ������£��й����ʵ������Ϣ�����
���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
�����µ��ܽ��/g | 36 | 109 | 21.5 | 9.6 |
������ϡ��Һ��pH | 7 | 13 | 11 | 9 |
�����ʵ������Ϣ��֪��ԭ��Һ������һ������________��
������ʵ�飩
��1��ȡ������Ʒ�������Һ��pH����7����ԭ��Һ�����ʿ϶�����________��
��2��ͬѧ����ȡ��Ʒ�ֽ���������ʵ�飬ʵ��������£�
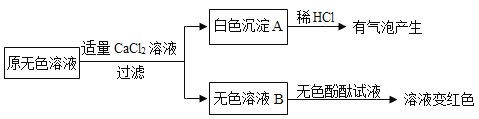
�����ɰ�ɫ����A�Ļ�ѧ����ʽ________��
��ͨ��ʵ������ж���Ʒ��ɫ��Һ�к��е�������________��
����ý��ۣ�
ʵ����ɺ����յõ��Ľ��ۣ�ԭƿ�е�������________�����ѱ��ʡ�
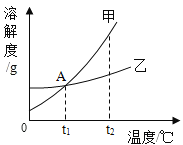
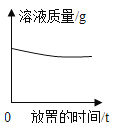
����Ŀ����1�������������£���100mL�Ȼ��Ʊ�����Һ������ͼ��ʾ������
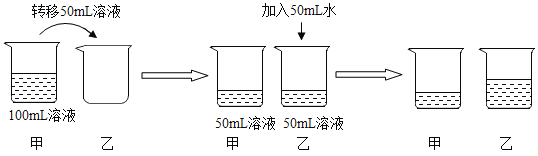
a.���ռס������ձ�����Һһ���DZ�����Һ����_____��
b.����Һ�ڼ�50mLˮ�Ĺ����У�������Щ����С����_____��
���ܽ�� �������������� ���ܼ������� ����Һ���ܶ�
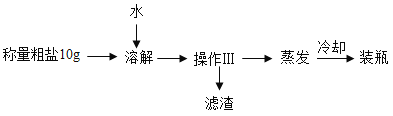
��2��������������ɳ�Ĵ�����Ʒ��ʵ����̺��Ȼ��Ƶ��ܽ���������£�
�¶ȣ��棩 | �ܽ�ȣ�g�� |
20 | 36.0 |
40 | 36.6 |
60 | 37.3 |
��20��ʱ�Ȼ��Ƶ��ܽ����_____
�ڸ�ʵ����������ɳ������ˮ���Ȼ���_____�����ʽ����ᴿ���������������_____��
�۹�������������ȷ����_____��ѡ���ţ���
a��������ͨ�����ȵķ�����ˮ������ȥ
b����������Ϊ�ƾ��ơ��������¶ȼ�
c��������Է�ֹ����������Һ�ηɽ�
d�����ȹ��������Թܼм�ס�������ƶ���ʹ���������
���ܽ�ʱ������ˮ�ĺ��ʵ���ԼΪ_____��ѡ����15������30�� ����60����mL��