��Ŀ����
����Ŀ����֪����ͭ��Ʒ����Ҫ�ɷ���CuO���������������ʡ�Ϊ�˲ⶨ��Ʒ������ͭ��������������ȤС���ͬѧ����������ʵ�飬���������̷�����
������1�����ᾧ�壨H2C2O4��3H2O����ŨH2SO4���������ȷֽ⣬��ѧ����ʽΪ��
H2C2O4��3H2O ![]() CO2��+ CO��+ 4H2O
CO2��+ CO��+ 4H2O
������2��Ũ���������ˮ�ԣ��dz�����Һ̬���������ʯ������ʯ�Һ��������ƵĹ�������dz����ĸ������������ˮ�Ͷ�����̼��
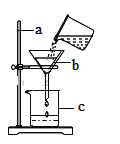
��1�����п���������ֽ���ȡ�����װ����__________������ĸ��ţ���
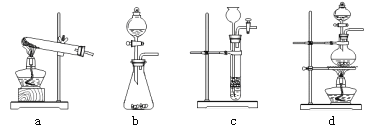
���������ۣ�����ͼ��ʾװ�ý���ʵ�飺
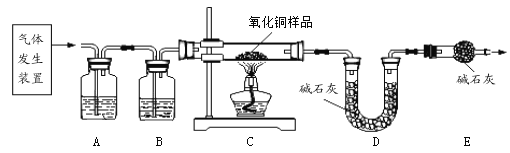
��2��ʵ��ǰӦ��_____________��
��3������C�е������Ǵ����������CO����A��B�е��Լ�������___________������ĸ��ţ���
a Ũ���� b �����ij����ʯ��ˮ
��4��Aװ�õ�������__________��д����Ӧ�ķ���ʽ_____________��
��5����Cװ�ü���ǰ��ֹͣ���Ⱥ�Ҫͨ�������CO�������÷ֱ��ǣ�
�ټ���ǰ_______________�� ��ֹͣ���Ⱥ�________________��
�����ݷ�������㣩
��ȡ����ͭ��Ʒ10.0g��������װ�ý���ʵ�飬��ȫ��Ӧ�Ƶ�Cװ����ʣ����������Ϊ8.4g����ԭ��Ʒ������ͭ����������______________����д��������̣�
��ʵ�����ۣ�
��˼1����ָ�����������ۣ���ʵ��װ�õ�һ������ȱ��__________��
��˼2�� ��ʵ�������ȱ��Bװ�ã��������������أ���������Ʒ������ͭ������������ʵ��ֵ��Ȼ�__________������ƫС����������������ƫ��������
��˼3����������������ͨ���ⶨ________ װ�ã�����ţ��������仯��Ҳ���Ի������ͭ�����ķ�����
���𰸡�d ���װ�õ������� b a ��ȥ�����еĶ�����̼ CO2+Ca(OH)2=CaCO3��+H2O �ų�װ���ڵĿ�������ֹ������ը ��ֹ�ȵ�ͭ���±����� 80% ��β������װ�ã�û�з�Ӧ����һ����̼����Ⱦ���� ���� D����D��E��
��������
��1������ֽ���������ҪŨ����������ȣ��ǹ�����Һ���ڼ��ȵ������·�Ӧ�ģ����Է���װ��ѡd��
��2����ʵ��ǰӦ�ȼ��װ�õ������ԣ�
��3����Ϊ��Ӧͬʱ�����������̼��ˮ������Ҫ��Ũ��������ˮ���������ij���ʯ��ˮ�����ն�����̼������ˮ�����һ������A���Լ�Ϊ�����ij���ʯ��ˮ��B�е��Լ�ΪŨ���ᣬ�b a��
��4��Aװ�õ������dz�ȥ�����еĶ�����̼����صĻ�ѧ����ʽΪ��CO2+Ca(OH)2=CaCO3��+H2O��
��5���ڼ���ǰͨһ����̼��Ŀ�����ų�װ���ڵĿ�������ֹ������ը���ڼ��Ⱥ����ͨһ����̼��Ŀ���Ƿ�ֹ�ȵ�ͭ���±�������
[���ݷ��������]
��ԭ��Ʒ������ͭ����������Ϊx

![]() =
=![]()
x=80%
[ʵ������]
��˼1����ʵ������һ���Ե�ȱ������β������װ�ã�û�з�Ӧ����һ����̼����Ⱦ������
��˼2�����ʵ����ͨ������Cװ�õĹ���仯��������Ʒ������ͭ�����������ģ����ȱ��Bװ�ã�ˮû��ȥ���ᱻD���գ�����������C�У����Զ�ʵ����û��Ӱ�죬����ʵ��ֵ��Ȳ��䡣
��˼3����������������ͨ���ⶨDװ�÷�Ӧǰ��������仯Ҳ�ɻ������ͭ��������������ΪDװ�����ӵ���������һ����̼��ԭ����ͭ���ɵĶ�����̼���������ٸ��ݻ�ѧ����ʽ���м��㡣�����������п����еĶ�����̼��ˮ����Eװ�ã�Ҳ�ɳ���D��Eװ�õ������ӵ��������м��㣩