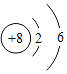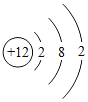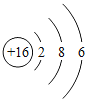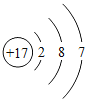��Ŀ����
����Ŀ��һЩ���������⣬ijС��ͬѧ�������ʵ�飬�Խ�����ʴ����̽����
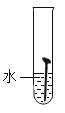
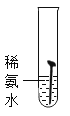
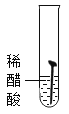
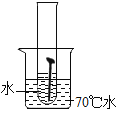
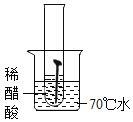
��ʵ��һ��ȡ5öȥ��ȥ��Ľྻ�������ֱ�װ���±����Թ��У�����ʵ�顣
ʵ��ͼʾ |
|
|
|
|
|
����ʱ�� | 8min | �ϳ�ʱ�䲻���� | 5min | 3min | 1min |
��1��ͨ������ʵ���֪���¶�Խ��������������Խ_____������������������������_____���������������������������������������������������ʽϿ졣
��ʵ�����Ϊ̽������ijɷ֣�����ͼ��ʾװ�ã��г�������ʡ�ԣ�����ʵ�飨ÿ����Ӧ�����վ���ȫ����
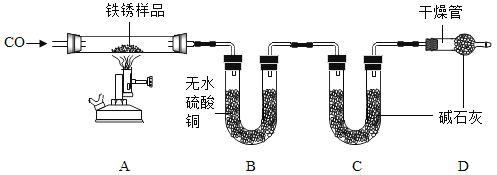
���������ϣ�����ˮ����ͭ������ˮ��������
�ڼ�ʯ�ҳɷ�Ϊ�������ƺ������ƣ���������ˮҲ������CO2��
��2����ָ����ʵ��װ�õ�һ��ȱ�ݣ�_____��
��3��A�м���ǰ����ͨ��COһ��ʱ�䣬Ŀ����_____��
��4����������Ʒ���ȣ���Ʒ��ڣ���ˮ����ͭ�������ɴ���֪������һ������_____Ԫ�أ��Ӷ��ƶϳ������е�_____�μ��������ⷴӦ��
��ʵ���¼��
������Ʒ������/g | װ��B������/g | װ��C������/g | |
��Ӧǰ | 23.2 | 232.4 | 198.2 |
��ַ�Ӧ�� | 11.2 | 239.6 | 211.4 |
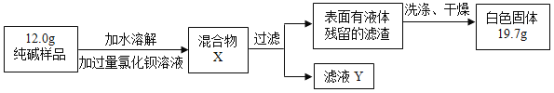
�����ϱ��������㣬��ȷ������ijɷ֣���FexOynH2O��ʾ����n=_____��
��5����ȱ��Dװ�ã�x:y��ֵ_____������ƫ��������ƫС��������Ӱ��������
��ʵ������С��ͬѧ��δ��ɰֽ��ĥ����������ʢ������ϡ������ܱ������У���ѹǿ�������������������ѹǿ�ͷ�Ӧʱ��ı仯������ͼ��ʾ��

��6���ش��������⣺
��ab�η�����Ӧ�Ļ�ѧ����ʽ��_____��
��bc�η�����Ӧ�Ļ�ѧ����ʽ��_____��
��cd��ѹǿ�仯����Ҫԭ����_____��
���𰸡��� ���� ��β������װ�ã������һ����̼��Ⱦ���� �ų�װ���еĿ�������ֹ������ը �� ˮ����H2O�� 4 ƫС Al2O3+6HCl=2AlCl3+3H2O 2Al+6HCl=2AlCl3+3H2�� �������¶��½���������������ѹ��С
��������
��1��ͨ������ʵ���֪���¶�Խ��������������Խ�죻
�����Ի����������������ʽϿ졣
����죻���ԡ�
��2����ʵ��װ�õ�һ��ȱ����û�д���β����һ����̼��ɢ����������Ⱦ������
�����β������װ�ã������һ����̼��Ⱦ������
��3��A�м���ǰ����ͨ��COһ��ʱ�䣬Ŀ�����ų�װ���еĿ�������ֹ������ը��
����ų�װ���еĿ�������ֹ������ը��
��4����������Ʒ���ȣ���Ʒ��ڣ���ˮ����ͭ������˵����Ӧ������ˮ���ɴ���֪������һ��������Ԫ�أ��Ӷ��ƶϳ������е�ˮ�μ��������ⷴӦ��
����⣻ˮ����H2O����
��Ʒ����Ԫ��������11.2g��FexOy����Ԫ������Ϊ��23.2g-��239.6g-232.4g��-11.2g=4.8g��
����������56x��16y=11.2g��4.8g��
x��y=2��3��
����������![]() =
=![]() ��n=4��
��n=4��
�������ijɷ���Fe2O34H2O
���4��
��5����ȱ��Dװ�ã������е�ˮ�����Ͷ�����̼�ᱻCװ�����գ����¶�����̼����ƫ�Ӷ�������������Ԫ������ƫ��һ������x��y��ֵƫС��
���ƫС��
��6����ab���У���������ϡ���ᷴӦ�����Ȼ�����ˮ��������Ӧ�Ļ�ѧ����ʽ�ǣ�Al2O3+6HCl=2AlCl3+3H2O��
���Al2O3+6HCl=2AlCl3+3H2O��
��bc���У�����ϡ���ᷴӦ�����Ȼ�����������������Ӧ�Ļ�ѧ����ʽ�ǣ�2Al+6HCl=2AlCl3+3H2����
���2Al+6HCl=2AlCl3+3H2����
��cd��ѹǿ�仯����Ҫԭ�����������¶��½���������������ѹ��С��
����������¶��½���������������ѹ��С��

����Ŀ��ijУ��ѧ��ȤС��ι��Ƽ���������Ϣ���������������о�������ԭ��:NaCl + NH3 + CO2+ H2O = NaHCO3��+ NH4C1�����������þ���A�� ��ʹA�������ȣ��ɷֽ��Ƶô��
��1�����ʵ����鴿����Ʒ���Ƿ���о���A��������±�(������װ����ѡ��):
ѡ���װ�� | ʵ������ | ʵ����� |
__________ | __________ | ��Ʒ��������A |
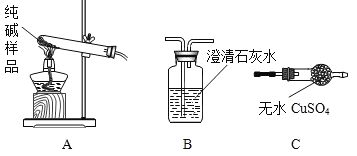
��2����ȡ������Ʒ��ˮ�ܽ⣬�����Һ�м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ_______________���ɴ�ȷ��������Ʒ��������NaCl��
�����̽������
��3��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ��:
�ټ���������Ȼ�����ҺĿ����___________________��
���ж������Ƿ�ϴ�ɾ�����������ϴ��Һ�еμ�________��Ȼ��۲������жϡ�
A�Ȼ�����Һ Bϡ���� Cϡ���� D̼�����Һ
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ__________��(��ȷ��0. 1 % )
����Ŀ����ѧ���ڷ��ӡ�ԭ��ˮƽ���о����ʵ���ɡ��ṹ�����ʼ���仯����һ�Ż�����Ȼ��ѧ���ش��������⣺
��1���ڶ�����̼�����������������������У�����������_____���ɣ���д�������ӵ����ƣ���
��2���±��г��˲���Ԫ�ص�ԭ�ӽṹʾ��ͼ���ش��������⣺
O | Mg | S | Cl |
|
|
|
|
��ԭ���ڻ�ѧ��Ӧ������_____����õ�����ʧȥ�������ӣ���Ԫ�غ���Ԫ�ػ�ѧ���ʾ��������Ե�ԭ��������ԭ�ӵ�_____��ͬ��
��3�����ж������е������÷������֪ʶ���Ͳ���ȷ����_____��
A���ڲ廨������Ʈ�㣬˵�����Ӳ��ϵ��˶�
Bˮ����ʱ��������ǣ�˵�����ӵĴ�С���¶����߶�����
C10mL�ƾ���l0mLˮ��Ϻ����С��20mL��˵������֮���м��
Dʪ�·����ڻ�¯�ԣ��ɵýϿ죬˵�������˶��������¶����߶�����
��4���ڻ�ѧ�仯ǰ��һ�������ı����_____������ĸ����
AԪ�ص����� Bԭ�ӵĸ��� Cԭ�ӵ����� D���ӵ�����
��5����Ԫ�غ���Ԫ���γɵĻ������ˮ��Һ������ᣨHF���������ڵ�̲���������Ҫԭ���������Ͳ�������Ҫ�ɷֶ������跢����Ӧ�������ķ����������ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ_____��