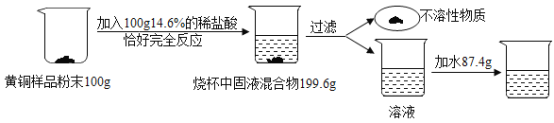
��Ŀ����
����Ŀ��̼�����һ����Ҫ����ԭ�ϣ��㷺���ڽ�������ֽ��ʳƷ��ҽҩ�ȹ�ҵ��
�� �ߴ�̼��Ƶ��Ʊ�
һ���Ե�ʯ��[��Ҫ�ɷ�ΪCa(OH)2������������MgO������]Ϊԭ���Ʊ��ߴ�CaCO3���������£�
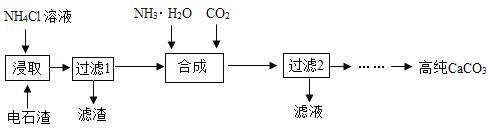
��1������ȡ������ҪĿ���ǽ�Ca(OH)2ת���CaCl2��ͬʱ�������а�ζ�����壩������ȡ��ʱ��Ӧ�Ļ�ѧ����ʽΪ_____��
��2��������2���õ�����Һ��һ�����е�������_____���ѧʽ����
�� ̼��Ƶ�Ӧ��
��3������ʯ��Ҫ�ɷ�ΪCaCO3��������������ĺ����ʵ�����ô���ʯ��ϡ���ᷴӦ�Ʊ�CO2���塣����װ�ÿ�����CO2������ᴿ���
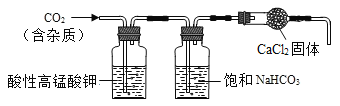
��װ���е����Ը���������ڳ�ȥCO2�����еĺ��������NaHCO3��Һ�����ڳ�ȥCO2�����е�______��CaCl2�������ڸ���CO2��
��ʵ���ҳ���______����CO2���壬ʵ������Ϊ_____��
��4����ҵ����ʯ��ʯ�����շ����еĶ���������ȡʯ�ࣨ��Ҫ�ɷ�Ϊ����ƣ�����������ͼ��

��֪��Ӧ��������ķ�ӦΪ��2CaCO3+2SO2+O2=2CaSO4+2CO2��
��������ʯ��ʯ��ˮ�Ƴ�ʯ�ҽ���Ŀ����_____��
����Ӧǰ����Ԫ�صĻ��ϼ�______����������������������������Ԫ�صĻ��ϼ�______���������������������������÷�Ӧ����������ԭ��Ӧ��
�����ٶ���������ŷţ���ҪΪ��______������ţ���
A ��������ЧӦ B ���������γ� C ��ֹ�ƻ�������
����������������2000�ַ����еĶ�������������Ҫ��5��̼��Ƶ�ʯ��ʯ���������ж����������������Ϊ______����д��������̣�
���𰸡�2NH4Cl+Ca(OH)2�TCaCl2+2NH3��+2H2O NH4Cl��дCa(HCO3)2Ҳ���֣� ��ȥCO2�����е�HCl���� ����ʯ��ˮ��������������Һ�� ����ʯ��ˮ����� ���Ӷ���������̼��ƵĽӴ������ʹ������������գ���1����֣� ���� ���� B 0.16%
��������
��ߴ�̼��Ƶ��Ʊ�
���̵�ʯ������Ҫ�ɷ�ΪCa(OH)2������������MgO�����ʣ����Ȼ����Һ��ϻ������Ȼ��ơ�ˮ�Ͱ����������ʹ��˵����õ��Ȼ�����Һ���ټ��백ˮ��ͨ�������̼ʹ�ø�������̼�����ʽ�������������˾���ϴ�ӡ�����Ȳ��輴�õ��ߴ��ȵ�̼��ơ�
��1������ȡ������ҪĿ���ǽ�Ca(OH)2ת���CaCl2��ͬʱ�������а�ζ�����壩������ȡ��ʱ��Ӧ�Ļ�ѧ����ʽΪ��2NH4Cl+Ca(OH)2�TCaCl2+2NH3��+2H2O�����2NH4Cl+Ca(OH)2�TCaCl2+2NH3��+2H2O
��2��������2���õ�����Һ��һ�����е�������NH4Cl��Ca(HCO3)2�����NH4Cl
�� ̼��Ƶ�Ӧ��
��3����װ���е����Ը���������ڳ�ȥCO2�����еĺ��������NaHCO3��Һ�����ڳ�ȥCO2������HCl���壬CaCl2�������ڸ���CO2��
��ʵ���ҳ��ó���ʯ��ˮ��������������Һ������CO2���壬ʵ������Ϊʯ��ˮ����ǣ���֤���ж�����̼�������ȥCO2�����е�HCl���壻 ����ʯ��ˮ��������������Һ��������ʯ��ˮ�����
��4������ˮ��ʯ��ʯ��ĩ����֮��ɵ�ʯ��ʯ����������������ͨ���Ϳ���ͨ���Ӧ�������з�����Ӧ��2CaCO3+2SO2+O2=2CaSO4+2CO2��������������õ�ʯ�ࡣ
��������ʯ��ʯ��ˮ�Ƴ�ʯ�ҽ���Ŀ�ģ������Ӷ���������̼��ƵĽӴ������ʹ������������ա�
�ڷ�Ӧǰ����Ԫ�صĻ��ϼ���SO2��+4�����ߵ�CaSO4�е�+6�ۣ���Ԫ�صĻ��ϼ��������е�0�۽��͵�CaSO4�е�-2�ۣ��÷�Ӧ����������ԭ��Ӧ��������ߣ�����
�۶��������ڿ����������γ����꣬�ʼ��ٶ���������ŷţ���ҪΪ�˼���������γɣ���B�������⡣��ѡB
�ܽ⣺������к��еĶ������������ΪX��
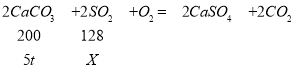
![]()
X=3.2t
�����ж����������������Ϊ��![]() ��
��
�𣺷����ж����������������Ϊ0.16%�����0.16%

����Ŀ��NaNO3��KNO3���ܽ�ȱ����ܽ���������¡�����˵����ȷ���ǣ� ��
�¶�/�� | 20 | 30 | 50 | 60 | 80 | |
�ܽ��S/g | NaNO3 | 87.6 | 94.9 | 110 | 122 | 148 |
KNO3 | 31.6 | 45.3 | 85.5 | 110 | 167 | |
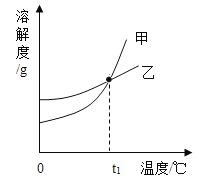
A.�ױ�ʾNaNO3�ܽ������
B.40 ��ʱ��������Һ��������������KNO3��NaNO3
C.t1Ӧ����60 �桫80 ��
D.80 ��ʱ��KNO3������Һ�к�������NaNO3��Ҫ�õ��ϴ�����KNO3���壬�ɲ��������ᾧ�����˵Ȳ���