��Ŀ����
����Ŀ��ѧϰ��ѧ���������ǵ�ѧ������������˵������ȷ����
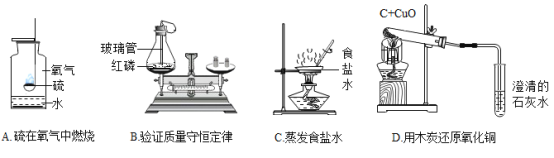
A.�仯���غ㣺2NaCl + 2H2O![]() 2X + Cl2��+ H2���У�X �Ļ�ѧʽ�� NaOH
2X + Cl2��+ H2���У�X �Ļ�ѧʽ�� NaOH
B.ģ�������������ý�����ϡ���ᷴӦ���������������ϡ���ᷴӦ���������ɵ�һ ���ǻ��ý���
C.������ۣ�����̿����ʹ��īˮ��ɫ��ԭ�������������ɶ�Ľṹ
D.ʵ����̽������ˮ���м������ˮ�������ֽ϶���ĭ��˵����ˮ������ˮ
���𰸡�B
��������
A�����ݻ�ѧ��Ӧǰ��ԭ�ӵ����ࡢ��Ŀ���䣬����֪X �Ļ�ѧʽ�� NaOH������ȷ��
B����ϡ���ᷴӦ����������IJ�һ���ǻ��ý�������̼���������ᷴӦ���ɶ�����̼���壬�ʴ���
C������̿�����ɶ�Ľṹ�������������ã��ܹ�����ɫ�غ���ζ�����Ի���̿��ʹ��īˮ��ɫ������ȷ��
D�������п��÷���ˮ������Ӳˮ����ˮ��������ĭ�϶������ˮ�����ٵ�Ӳˮ������ȷ��
��ѡB��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�����Ŀ��̼��þˮ�������Ʊ�þ��Ʒ���м��塣
��.����ȡMgCO33H2O����ҵ�ϴ�������±ˮ����Ҫ�ɷ�ΪMgCl2���л�ȡMgCO33H2O�ķ�����ͼ1��
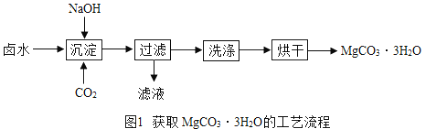
��1���������̵õ���MgCO33H2O������Ȼ��ƵĻ���д���������̷����Ļ�ѧ��Ӧ����ʽ��________________________________________________��
��2��������Ҫ�õ��IJ����������ձ�����������______��֤��MgCO33H2O������ϴ�Ӹɾ��ķ����ǣ�ȡ���һ��ϴ��Һ��_____________________________��˵����ϴ����
��3���������̵���Һ��þ���Ӻ�����ʱ��ı仯��ͼ3��ʾ����ͬ�¶������õ������������±���ʾ��
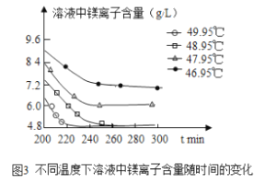
�¶ȣ��棩 | ���� |
46.95 | MgCO33H2O |
47.95 | MgCO33H2O |
48.95 | MgCO33H2O |
49.95 | Mg5��OH��2��CO3��44H2O |
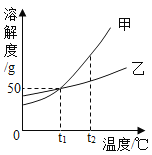
���������ѡ����¶�Ϊ________�������Ǵ��¶��£�Mg2+���������ʽϿ죬����Ч�ʽϸߣ��Ҳ�����������������
��.���ⶨMgCO33H2O�Ĵ��ȣ�
���������ϣ�
a.��ʯ����CaO��NaOH�Ĺ������
b��MgCO33H2O+H2SO4=MgSO4+CO2��+4H2O��
c��Mg5��OH��2��CO3��44H2OҲ���Ա�ʾΪMg��OH��24 MgCO34H2O������Է�������Ϊ466���������ᷴӦ����CO2��
��ʵ�鲽�裩
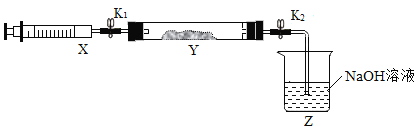
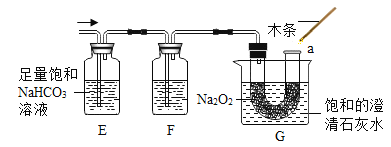
��������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ȷ��MgCO33H2O�Ĵ��ȡ�
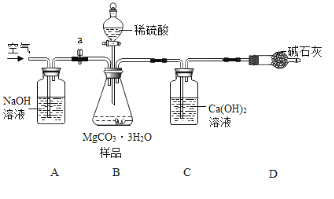

����ʵ����̻ش��������⣺
��1��ʵ����������������ͨ�������������Ϊ______________________________��
��2��Cװ���з�Ӧ����CaCO3�Ļ�ѧ����ʽΪ_______________________��D�м�ʯ�ҵ�����Ϊ_______________________________��
��3�����и����ʩ�У�����߲ⶨȷ�ȵ���_________�����ţ���
a�ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
bΪ������ʵ��ʱ�䣬���ٵμ�����
c��B��C֮������ʢ��Ũ�����ϴ��װ��
d��Cװ����ർ��ĩ�������������
e��Cװ���г���ʯ��ˮ����Ba��OH��2��Һ
��4��ʵ����ȷ��ȡ15.0g��Ʒ���ݣ��������βⶨ���������CaCO3������ƽ������Ϊ10.0g���������Ʒ��MgCO33H2O�Ĵ��ȣ�д��������̣���______
��5������ȡMgCO33H2O����Ʒ�к�������Mg5��OH��2��O3��44H2O������Ʒ��MgCO33H2O�Ĵ���___������ƫ����������������ƫС������