��Ŀ����
����Ŀ����ˮ�к��д������Ȼ��ƣ���ҵ�ϳ��þ��Ƶ��Ȼ���Ϊԭ���Ʊ��������ش���������:
(1)�����ʵ�Ԫ������Ϸ�������Ҫ���뺬______Ԫ�ص����ʣ���ʵ�����������У�ͨ��������ʳ��ˮ��ͨ�백�����Ƴɱ��Ͱ���ˮ��ԭ����______________________________________��
(2)��ҵ���ƵõĴ����г������������Ȼ���(��������������)��ij��ѧ��ȤС���ͬѧҪ�ⶨij���������Ĵ�����Ʒ��̼���Ƶ�����������������·���:����һ:�ס��ҡ�����λͬѧ�քe��ȡһ����������Ʒ�����ձ��У���������ˮ������Һ�м���һ���������Ȼ�����Һ����ַ�Ӧ����ˣ�������ϴ�ӡ����ﲢ���������ʵ�����ݼ�¼���±���ʾ��
������Ŀ | �� | �� | �� |
��ȡ��Ʒ������/g | 12 | 12 | 15 |
�����Ȼ�����Һ������/g | 150 | 100 | 100 |
��Ӧ�����ó���������/g | 10 | 10 | 10 |
����������������ݣ��������Ʒ��̼���Ƶ�����������(д�����㲽�裬������������0.1%)______��
�ڷ�����:��ͬѧ�õ�����ƽ�ֱ��ȡ12g��ͬ��̼������Ʒ��100gϡ���ᣬ��ַ�Ӧ����ձ���ʣ�����ʵ�����Ϊ108.6g�����ͬѧ��õ�̼���Ƶ���������_________(����ڡ���С�ڡ������ڡ�)����һ�в�õ�̼���Ƶ�����������ԭ�������_________________��(����װ������������)
���𰸡�̼���� ������ˮ��Һ�Լ��ԣ�������̼��ˮ��Һ�����ԣ�����������кͷ�Ӧ 88.3�� С�� ����ϡ�������������
��������
(1)�Ȼ����к�����Ԫ�غ���Ԫ�أ�̼�����к���̼Ԫ�ء���Ԫ�غ���Ԫ�أ������ʵ�Ԫ������Ϸ�������Ҫ���뺬̼Ԫ�غ���Ԫ�ص����ʣ���ʵ�����������У�ͨ��������ʳ��ˮ��ͨ�백�����Ƴɱ��Ͱ���ˮ��ԭ���ǣ�������ˮ��Һ�Լ��ԣ�������̼��ˮ��Һ�����ԣ�����������кͷ�Ӧ��
(2) ������Ʒ��̼���Ƶ�����Ϊx��
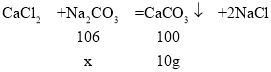
![]()
x=10.6g
��Ʒ��̼���Ƶ���������=![]() ��100����88.3��
��100����88.3��
�ڷ����������ɶ�����̼���������=12g+100g-108.6g=3.4g
�跽��һ�е�������̼������Ʒ��������ϡ���ᷴӦ���ɶ�����̼������Ϊy��

![]()
y=4.4g
4.4g��3.4g
�ʷ������и�ͬѧ��õ�̼���Ƶ���������С�ڷ���һ�в�õ�̼���Ƶ�����������ԭ������ǣ�����ϡ������������㡣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij��ѧ��ȤС����һ��ʵ���з���þ���ƶ����ڴ����Ķ�����̼������ȼ�գ���Ӧ����ȴ��ƿ�׳����к�ɫ������ƿ���ϻ������Ű�ɫ���ʡ������飬��ɫ������̼�����Ƕ�þ�����ڶ�����̼������ȼ�պ����ɵİ�ɫ���ʷֱ����������̽����
��̽������һ��þ�ڴ����Ķ�����̼��ȼ�����ɵİ�ɫ������ʲô��
ͬѧ�������ų�����Mg(OH)2�Ŀ����ԣ�������_____��
���������룩��ɫ��������Ǣ�MgO����MgCO3����_____��
������ʵ�飩ȡ������ɫ���壬����������ϡ���ᣬ�۲쵽_____���������ȷ��
��ʵ����ۣ�þ�ڶ�����̼��ȼ�յĻ�ѧ����ʽ��_____��
��̽������������ڴ����Ķ�����̼��ȼ�����ɵİ�ɫ������ʲô��
���������ϣ���Ϣ1����������ˮ�������ҷ�Ӧ��
��Ϣ2��Na2O+H2O=2NaOH
��ʵ����ƣ����鷽����
ʵ�鲽�� | ʵ������ | ���� |
ȡ������ɫ�������Թ��У���ˮ�ܽ⣬��������Ba(OH)2��Һ | _____ | ��ɫ��������Na2CO3 |
���ú����ϲ���Һ�μӷ�̪��Һ | ��Һ��� | ��ɫ��������Na2O |
������ۣ���ɫ����ΪNa2O��Na2CO3�Ļ����
���鷽����
ȡ5.3�˰�ɫ������Ʒ�������в�����
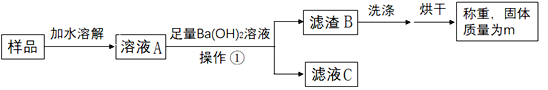
��1����������_____��
��2��Ba(OH)2��Һ����������Ŀ����_____��
����ͬѧ���m=9.85�ˣ������Ʒ�������з��������յó����ۣ���ɫ����ΪNa2CO3��
��ʵ�鷴˼��
��1��Ϊ�μס����������ý��۲������ijͬѧ������ָ�����鷽������ѧ��Ӧ�ý����鷽���е�Ba(OH)2��Һ��Ϊ_____��Һ�ź�����
��2�����ڶ�����̼��ȼ�յĻ�ѧ����ʽΪ_____��
����չӦ�ã�
ʵ�������ƵȽ�����ȼ����������ʱ������ѡ������ʽ��_____��
A����ˮ���� B����Һ̬������̼����� C����ɳ������