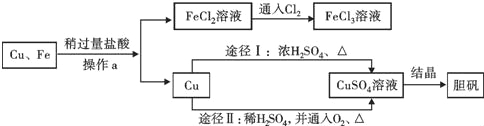
��Ŀ����
����Ŀ��С����Э����ʦ����ʵ�鴢����ʱ������һƿ��Ŷ�����������ơ�Ϊ������������������������̽����ȡ����������Ʒ11.4g����ƿ�У�����38.6gˮ������ƿ����εμ�14.6%��ϡ���ᣬ����ϡ�������������ƿ�����ʵ�������ϵ��ͼ��ʾ��

��1���������Ʊ��ʵ�ԭ����_____���û�ѧ����ʽ��ʾ����
��2�����������Ʒ�Ӧ��ϡ������Һ������Ϊ_____g��
��3������11.4g����Ʒ���������Ƶ�������_____
��4�����ݼ����������ݻ�������CO2�����������ߣ������б�Ҫ�ı�ע����_____
���𰸡�Ca��OH��2+CO2=CaCO3��+H2O 50 7.4g 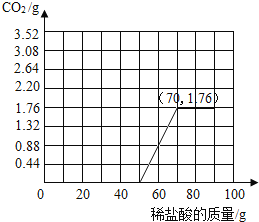
��������
��1������������������еĶ�����̼��Ӧ�������������Ʊ��ʵ�ԭ��Ӧ�Ļ�ѧ����ʽ�ǣ�Ca��OH��2+CO2=CaCO3��+H2O��
��2���������ƺ�̼��ƵĻ���������ᷴӦʱ�����������������ᷴӦ�����������������ᷴӦ���̼����������ᷴӦ����ͼ���֪�����������Ʒ�Ӧ��ϡ������Һ������Ϊ50g��
��3���裺�����ᷴӦ��Ca��OH��2������Ϊx
![]() ��ã�x=7.4g��
��ã�x=7.4g��
��4����Ʒ��̼��Ƶ�����=11.4g-7.4g=4g��
�裺4g̼�����ϡ���ᷴӦ����CO2������Ϊy�����������Ϊz��

![]()
��ã�y=1.76g z=20g
�����������֪������CO2�����������ߵ�����ǣ�50��0����ת�۵�Ϊ��70��1.76����ͼ������ͼ��
 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������һ�ֻ��ý���������������������ˮ�����ʷ�����Ӧ������ˮ��Ӧ�Ļ�ѧ����ʽΪ:2Na+2H2O==2NaOH+H2������ʵ���ң�С����ȡ����������ͭ��Һ���ձ��У���ȡһС����Ͷ������ͭ��Һ�У���ַ�Ӧ����ˣ��õ���Һ����ɫ�����������˱��ijɷֽ���̽����
�������������Һ�����ʵijɷ���ʲô?
������������Na2SO4��Һ������
�����������������һ:Na2SO4
�����:Na2SO4��NaOH
������:____________
������:Na2SO4��CuSO4��NaOH
С����Ϊ����______��������������_______(�û�ѧ����ʽ��ʾ)��
��ʵ������֤��
ʵ�� | ʵ����� | ʵ��F�� | ʵ����� |
ʵ��һ | ȡ������Һ���Թ��У���ϸ�۲����� | ��ҺΪ��ɫ | ����_____������ |
ʵ��� | ��ʵ��һ��ȡ��Һ�е����̪��Һ | ________ | ����������� |
ʵ���� | �����£���ȡ������Һ������Һ���� | pH=7 | ����_____���� |
����չ�������������˳������Ƽ�ǰ��Ľ���____(������������������)��λ�ں���Ľ�������������Һ���û�������
����Ŀ��������ͬѧ�Dzⶨ��ͭ��ͭп�Ͻ���ͭ�����������Ĺ��̡�
��1��С��ͬѧȡ10 g��ͭ������������ϡ���ᣬ���ռ�������0.2 g�����ͭ��ͭ�����������Ƕ���__________����д��������̣�
��2��С��ͬѧ��ȡ10 g��ͭ���ձ��У���120 gϡ��������μ����ձ��У���ü����ϡ�����������ձ���ʣ������������±�������m=___________��
���� | һ | �� | �� | �� | �� | �� |
����ϡ���������/g | 20 | 20 | 20 | 20 | 20 | 20 |
�ձ���ʣ���������/g | 29.96 | 49.92 | m | 89.84 | 109.8 | 129.8 |