��Ŀ����
�������Ĵ����Ʒ�������Ậ���������Ȼ��ƣ�Ϊ�˲ⶨ����ɣ�ijУ��ѧ��ȤС���ͬѧ�������������ʵ�飺
��1�����Թ�ȡ������Ʒ���������м������ϡ���ᣬ�ٵ���������������Һ�����۲쵽______����֤������Ʒ�к����Ȼ��ƣ�
��2��Ϊ�ⶨ�ò�Ʒ��̼���Ƶĺ�����������ͼ����ʵ�飺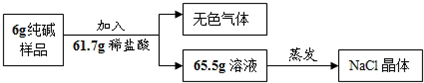
�ٸ��������غ㶨�ɣ���ʵ��������ɫ�����������______g��
�ڼ����6g������Ʒ��̼���Ƶ���������д��������̣�����������ȷ��0.1g��
�����������þ����������м��㣬���������______�����ƫ����ƫС������Ӱ�족��
��1�����Թ�ȡ������Ʒ���������м������ϡ���ᣬ�ٵ���������������Һ�����۲쵽______����֤������Ʒ�к����Ȼ��ƣ�
��2��Ϊ�ⶨ�ò�Ʒ��̼���Ƶĺ�����������ͼ����ʵ�飺

�ٸ��������غ㶨�ɣ���ʵ��������ɫ�����������______g��
�ڼ����6g������Ʒ��̼���Ƶ���������д��������̣�����������ȷ��0.1g��
�����������þ����������м��㣬���������______�����ƫ����ƫС������Ӱ�족��
��1�����Թ�ȡ������Ʒ���������м������ϡ���ᣬ�ٵ���������������Һ�������ְ�ɫ��������֤������Ʒ�к��������ӣ����ڸ��⣬�������Ȼ��ƣ�
�ʴ�Ϊ�����ְ�ɫ������
��2���ٸ��������غ㶨�ɣ���Ӧǰ����ٵ�����Ϊ���ɵĶ�����̼������������6g+61.7g-65.5g=2.2g��
�ʴ�Ϊ��2.2g��
����Na2CO3����Ϊx����
Na2CO3+2HCl=2NaCl+H2O+CO2����1�֣�
10644
x2.2g
=
���x=5.3g
�𣺸���Ʒ�к���5.3gNa2CO3��
�����þ�������������Ӧ���ɵ��Ȼ��Ƶ�������ԭ��������Ȼ��Ƶ����������������þ����������м��㣬���������ƫ��
�ʴ�Ϊ��ƫ��
�ʴ�Ϊ�����ְ�ɫ������
��2���ٸ��������غ㶨�ɣ���Ӧǰ����ٵ�����Ϊ���ɵĶ�����̼������������6g+61.7g-65.5g=2.2g��
�ʴ�Ϊ��2.2g��
����Na2CO3����Ϊx����
Na2CO3+2HCl=2NaCl+H2O+CO2����1�֣�
10644
x2.2g
| 106 |
| 44 |
| x |
| 2.2g |
���x=5.3g
�𣺸���Ʒ�к���5.3gNa2CO3��
�����þ�������������Ӧ���ɵ��Ȼ��Ƶ�������ԭ��������Ȼ��Ƶ����������������þ����������м��㣬���������ƫ��
�ʴ�Ϊ��ƫ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ