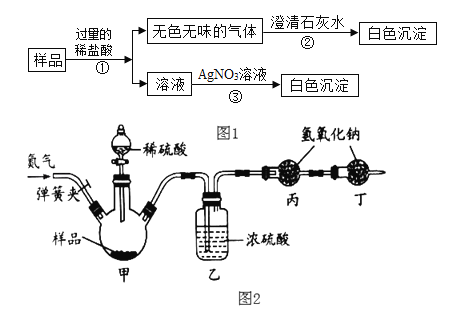
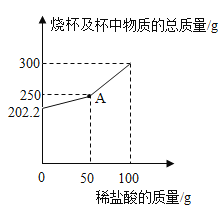
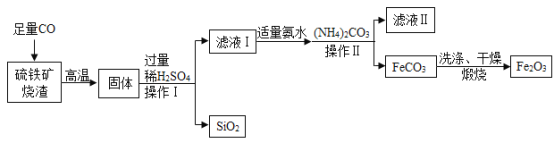
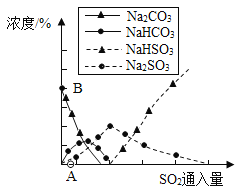
��Ŀ����
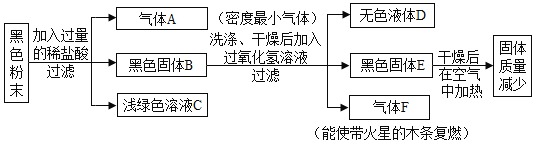
����Ŀ����۶�άͼ��ӳ����Ԫ�صĻ��ϼ����������֮��Ĺ�ϵ��ͼ 1 ��ijͬѧ���ƵĹ���̼Ԫ�ص���۶�άͼ��
��1��a ���⻯��Ϊ��Ȼ������Ҫ�ɷ֣�����ȫȼ�յĻ�ѧ����ʽΪ_____________��
��2��b ����Ӧ���ʵ������_________________��������;��______________��
��3��ú����Ҫ�ɷ��ǵ���̼��ͼ 2 ����ú�ϳ�ˮú���ķ�Ӧ��ʾ��ͼ���÷�ӦΪ�û���Ӧ���������Ļ�ѧʽΪ_____________���μӷ�Ӧ�ļ����ɵı���������Ϊ_____________��
��4���������� CO �ж��� CO2 ����CO ��ȼ�ն� CO2 �����������۵ĽǶȽ������ǻ�ѧ���ʲ�һ����ԭ��_____________��
���𰸡�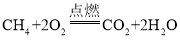 �� �����ͷۡ�����θ������ H2 18��28 ���ӵĹ��ɲ�ͬ
�� �����ͷۡ�����θ������ H2 18��28 ���ӵĹ��ɲ�ͬ
��������
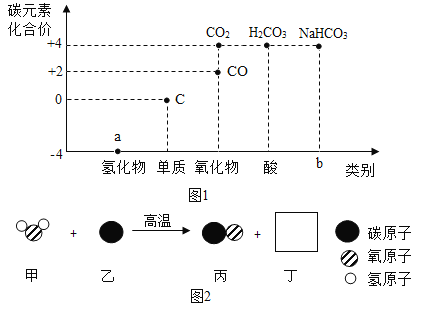
��1��a���⻯��Ϊ��Ȼ������Ҫ�ɷ֣����⻯��Ϊ���飬�仯ѧʽΪ��CH4����ȫȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ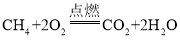 ��
��
��2��b ��̼�����ƣ������Σ����������ͷۡ�����θ����ࣻ
��3���������غ㶨�ɣ���ѧ��Ӧǰ��ԭ�ӵ����༰��Ŀ�����֪���÷�Ӧ�еĻ�ѧ����ʽΪ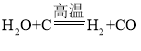 �����Զ��Ļ�ѧʽΪH2���μӷ�Ӧ�ļ����ɵı���������Ϊ18��28=9:14��
�����Զ��Ļ�ѧʽΪH2���μӷ�Ӧ�ļ����ɵı���������Ϊ18��28=9:14��
��4��һ����̼�������̼�����ʵ����Ԫ����ͬ��CO�ж�����CO2��������Ϊ���ӵĹ��ɲ�ͬ��

����Ŀ����ѧ��ȤС������������ʵ��̽�����
[̽��һ]�����������
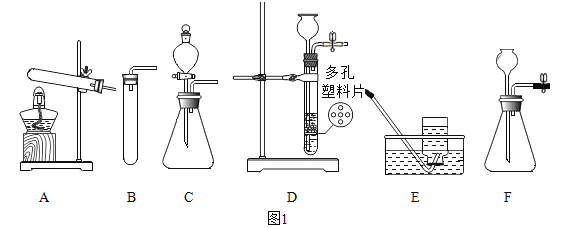
[ʵ�� 1]���ྻ�������˿������ͼһ��ʾװ���У��ر�ֹˮ�� K�� ����һ��ʱ��۲죬װ���е���˿û���⼣��
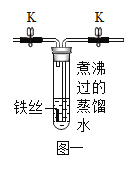
��1������ˮ������е�Ŀ����_______________��
��2����˿û���⼣˵����______________��
[ʵ�� 2]��ֹˮ�� K��ͨ�� O2���ų��Թ��е�ˮ���Թܵײ���������ˮ������ͼ����ʾ���ر�ֹˮ�� K������һ��ʱ��۲쵽��˿�������ء�
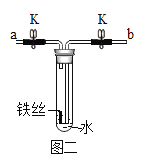
��3��ͨ�� O2 ʱ��Ӧ��__________�ˣ�����a������b����ͨ�롣
[ʵ�� 3]��ȤС����Ϊ������������ʵ����������Եó�����������������ˮ��ͬ���õĽ��ۣ���������ͼһװ�ò�����һ��ʵ�顣
��4��������ȤС�鲹��ʵ��ľ��巽�������۲쵽������______________��
[̽����] ������������������ʴ��Ӱ������
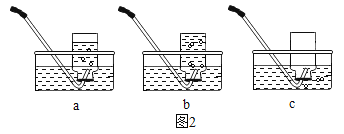
[ʵ�� 4]����Ͼ��ȵ����ۺ�̼��������ƿ�ײ�������ƿ������ͼ�������ӽ�ͷ�ι��е��뼸�δ�����Һ��ͬʱ���������е�ѹǿ�仯��
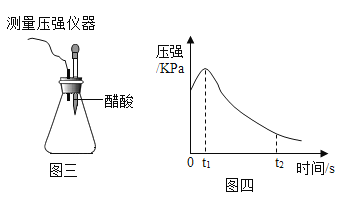
��� | ʵ��Ŀ�� | ̼��/g | ����/g | ����/% |
�� | Ϊ����ʵ�������� | 1.0 | 3.0 | 80.0 |
�� | ̽������Ũ�ȵ�Ӱ�� | 1.0 | 32.0 | |
�� | 0.5 | 3.0 | 80.0 |
���ϣ�̼�۲�������ƿ�еĻ�ѧ��Ӧ��������Һ�����ԡ�
��5���ϱ��б�Ţ�ʵ�������۵�����Ϊ_____________����Ţ������ʵ��Ŀ����___________��
��6����Ţ�������������ѹǿ��ʱ��仯��ͼ�ģ�t2 ʱ������ѹǿ����С����ʼѹǿ����ԭ����___________��
��7����ȤС���ܽ���ͼ���� 0��t1 ʱѹǿ�����ԭ������__________��
����չ���죩
��8���������Ҫ�ɷ��� Fe2O3������������������в����ĺ��ɫ���� Fe(OH)3 �ֽ�����ģ�д�� Fe(OH)3 ���ȷֽ��������������Ļ�ѧ����ʽ_____________��
��9��Ϊ�˷�ֹ������ʴ�����dz������������Ϳˢ�����ͻ�������������ȸ��DZ�����ķ�������Щ�������ܷ�ֹ��ʴ��ԭ����______________��
����Ŀ�����A��B��������ѡһ����������������𣬰�A�Ʒ֡�
A  ̽��������̼��ˮ��Ӧ
̽��������̼��ˮ��Ӧ
B 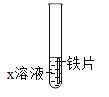 ̽�����Ľ������
̽�����Ľ������
A | B |
(1)ͼA̽��������̼��ˮ��Ӧ��ʵ�����Ϊ_______�� (2)��ʵ��Ľ�����_____(���û�ѧ����ʽ��ʾ)�� | (1)ͼB�У���X��ҺΪ����ͭ��Һ����Ӧ�Ļ�ѧ����Ϊ______�� (2)��X��ҺΪϡ���ᣬ�۲쵽�����ݲ������÷�Ӧ������ԭ����_____�� |