��Ŀ����
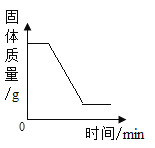
����Ŀ��Ϊ�ⶨпͭ�Ͻ���п�ĺ�����ȡ�úϽ������ͼ 14 ʢ��ϡ�������ƿ�У�������Ӧ�� Zn+H2SO4=ZnSO4+H2�� �����ʵ���ȡƽ��ֵ�����������±���

��Ӧǰ | ��ַ�Ӧ��װ�ü���Ӧʣ���������� | |
װ�ú�������ϡ�������� | пͭ�Ͻ����� | |
342.10g | 16.00g | 357.70g |
�������Ǹ�������տ����е�ˮ���������㣺
��1�����������غ㶨��������������������
��2���úϽ���п������������
���𰸡���1��0.4g ��2��81.25%
��������
�йػ�ѧ����ʽ�ľ��ǰ����йػ�ѧ����ʽ�ļ���IJ��裺(1)��δ֪����2����������д����ʽ��3��д���������4���б���ʽ��⣨5����д���𰸡������кϽ��ܹ�Ϊ16g ����ֻ��Zn��ϡ���ᷢ����Ӧ��ͨ����Ӧǰ�������ı仯���H2����������ͨ��H2���������Zn����������һ���ͳ�Zn������������
�⣺������ɵ�
��1������������=342.10g+16.00g��357.70g=0.4g
������������Ϊ0.4g��
��2���⣺��úϽ���п������Ϊx��

![]()
x=13g
��úϽ���п����������Ϊ![]() ��100%=81.25%
��100%=81.25%
�𣺸úϽ���п����������Ϊ81.25%��

��ϰ��ϵ�д�
�����Ŀ