��Ŀ����
�ᡢ������й㷺��;����Ҫ�����ij��ѧ�С���ͬѧΧ���⼸����������һϵ�е�̽�������1��ͬѧ�Ǵ�ʢŨ�����Ũ�����Լ�ƿ��ƿ�ǣ��������ܰ��������ֿ���������Ϊʲô��
��2����ͼ��ij�Լ�ƿ��ǩ�ϵ����ݣ�Ҫ��10g����Ũ����ϡ��Ϊ20%�����ᣬ��Ҫˮ������Ϊ
| ��Ũ���ᣨ�������� ��ѧʽ��H2SO4������Է���������98 �ܶȣ�1.84/cm3�� ����������98% |
��ȡ�����ù�����Ʒ�����Թ��У������м���һ����ɫ��Һ�����������ݲ�����˵������Ʒ�к���̼���ƣ��ɴ˿�ȷ���ù����ѷ������ʣ�����ɫ��Һ������
��Ϊ̽���ù������Ƿ���δ���ʵ��������ƣ�ͬѧ���ֽ��������±���ʾ��ʵ�飮��֪̼���Ƶ�ˮ��Һ�ʼ��ԣ����Ĵ��ڻ���������Ƶļ�����ɸ��ţ�
| ������ ������ |
OH- | NO3- | Cl- | SO42- | CO32- |
| H+ | �ܡ��� | �ܡ��� | �� | �ܡ��� | |
| Na+ | �� | �� | �� | �� | �� |
| Ba2+ | �� | �� | �� | ���� | ���� |
| ʵ��Ŀ�� | ʵ����� | ���� | ���ۻ�ѧ����ʽ |
| ��ȥ̼���� | ȡ�����ù�����Ʒ����ˮ�����Һ���μ������� |
�а�ɫ�������� | �йط�Ӧ�Ļ�ѧ����ʽΪ |
| �����Ƿ����������� | ����Һ�еμӷ�̪��Һ | ����Ʒ�к����������� |
��������1������Ũ����Ļӷ��ԣ�����Ũ�����Ũ���Ũ����ƿ�ڳ��ְ��������ŵ��̼������壮���ʾ���һ�������ԣ�
��2������ϡ��ǰ���������������������ϡ��ʱ����Ҫˮ�����������Ͳ�����
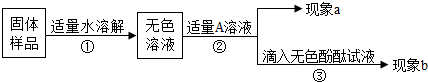
��3��̽��һƿ�������ƹ���ı�������������������տ�����Ķ�����̼����̼���ƣ�
������̼���ε����ʣ�����̼���ƵĴ��ڣ�֤���������Ʊ��ʣ�
��ѡ����ʵ���Һ��ȥ̼���ƣ��������������Ƿ���ڣ���ȥ̼����ʱ����ʹ�ú����ɼ������ʣ�����������������Ƶļ��飻
��4����ѧ����ʽ����Һ�����������������ϵļ��㣮�����û�ѧ����ʽ���м���ʱ��127g������������Һ�����������Բ��ܰ�127g��Ϊ�������Ƶ�������ʹ�ã���Ӧ�Ȳ�������������������Ӧ��������Һ������Ϊ����������Һ��ϡ������Һ�������ͣ�
��2������ϡ��ǰ���������������������ϡ��ʱ����Ҫˮ�����������Ͳ�����
��3��̽��һƿ�������ƹ���ı�������������������տ�����Ķ�����̼����̼���ƣ�
������̼���ε����ʣ�����̼���ƵĴ��ڣ�֤���������Ʊ��ʣ�
��ѡ����ʵ���Һ��ȥ̼���ƣ��������������Ƿ���ڣ���ȥ̼����ʱ����ʹ�ú����ɼ������ʣ�����������������Ƶļ��飻
��4����ѧ����ʽ����Һ�����������������ϵļ��㣮�����û�ѧ����ʽ���м���ʱ��127g������������Һ�����������Բ��ܰ�127g��Ϊ�������Ƶ�������ʹ�ã���Ӧ�Ȳ�������������������Ӧ��������Һ������Ϊ����������Һ��ϡ������Һ�������ͣ�
����⣺��1��Ũ�����ӷ����ӷ������Ȼ���������������ˮ������ϳ������СҺ�Σ�ƿ�ڳ��ְ�������Ũ����ӷ����������Ȼ�������Ĵ̼�����ζ����ƿ�����ŵ��̼�����ζ��ΪŨ���ᣮ
�ʴ�ƿ�ڳ��ְ�������Ũ���ᣬ��ΪŨ�����лӷ��ԣ������ŵ��̼�����ζ��ΪŨ���ᣩ
��2���ݱ�ǩ��ƿ��Ũ������������Ϊ98%������Ҫˮ������Ϊx
10g��98%=��x+10g����20% ��֮�� x=39g
�ʴ�39��
������ܶȽ�С��ˮ�����ܶȴ��Ũ�����ˮ����Ũ�������棬Ũ�����ܽ�ʱ�ų���������ʹˮ���ڣ������Һ�ɽ�
�ʴ�ˮ���ܶȽ�С������Ũ�������棬�ܽ�ʱ�ų����Ȼ�ʹˮ���ڣ������Һ�ɽ���
��3����̼���ο�����ϡ���ᷴӦ�ų���ʹ����ʯ��ˮ����ǵĶ�����̼���壻
�ʴ����ᣨ������ȣ�
�ڸ����ܽ��Ա�����ѡ��BaCl2��Ba��NO3��2��ȥ̼���ƣ��ų�̼���Ƶ�Ӱ��μӷ�̪��Һ�����������ƣ���ɫ��̪��죬֤����Һ�к����������ƣ�
��ѡBaCl2����Ba��NO3��2����BaCl2+Na2CO3=BaCO3��+2NaCl����ɫ��̪��죻
��4���⣺�跴Ӧ�������Ȼ��Ƶ�����Ϊx��
HCl+NaOH=NaCl+H2O
36.5 58.5
73g��20% x
=
��
��֮�ã�x=23.4g
��Ӧ��������Һ�����ʵ���������Ϊ
��100%=11.7%
�𣺷�Ӧ��������Һ�����ʵ���������Ϊ11.7%��
�ʴ�ƿ�ڳ��ְ�������Ũ���ᣬ��ΪŨ�����лӷ��ԣ������ŵ��̼�����ζ��ΪŨ���ᣩ
��2���ݱ�ǩ��ƿ��Ũ������������Ϊ98%������Ҫˮ������Ϊx
10g��98%=��x+10g����20% ��֮�� x=39g
�ʴ�39��
������ܶȽ�С��ˮ�����ܶȴ��Ũ�����ˮ����Ũ�������棬Ũ�����ܽ�ʱ�ų���������ʹˮ���ڣ������Һ�ɽ�
�ʴ�ˮ���ܶȽ�С������Ũ�������棬�ܽ�ʱ�ų����Ȼ�ʹˮ���ڣ������Һ�ɽ���
��3����̼���ο�����ϡ���ᷴӦ�ų���ʹ����ʯ��ˮ����ǵĶ�����̼���壻
�ʴ����ᣨ������ȣ�
�ڸ����ܽ��Ա�����ѡ��BaCl2��Ba��NO3��2��ȥ̼���ƣ��ų�̼���Ƶ�Ӱ��μӷ�̪��Һ�����������ƣ���ɫ��̪��죬֤����Һ�к����������ƣ�
��ѡBaCl2����Ba��NO3��2����BaCl2+Na2CO3=BaCO3��+2NaCl����ɫ��̪��죻
��4���⣺�跴Ӧ�������Ȼ��Ƶ�����Ϊx��
HCl+NaOH=NaCl+H2O
36.5 58.5
73g��20% x
| 36.5 |
| 58.5 |
| 73g��20% |
| x |
��֮�ã�x=23.4g
��Ӧ��������Һ�����ʵ���������Ϊ
| 23.4g |
| 73g+127g |
�𣺷�Ӧ��������Һ�����ʵ���������Ϊ11.7%��
���������������غ���㷴Ӧ����Һ�����Ƚ�ֱ�ۣ���Ӧ�Ȳ�������������������Ӧ��������Һ������Ϊ��Ӧǰ����Һ�������ͣ�

��ϰ��ϵ�д�
�����Ŀ
�ᡢ������й㷺��;����Ҫ�����ij��ѧ�С���ͬѧΧ���⼸����������һϵ�е�̽�����
��1��ͬѧ�Ǵ�ʢŨ�����Ũ�����Լ�ƿ��ƿ�ǣ��������ܰ��������ֿ���������Ϊʲô��
��2����ͼ��ij�Լ�ƿ��ǩ�ϵ����ݣ�Ҫ��30 g����Ũ����ϡ��Ϊ40%�����ᣬ��Ҫˮ������Ϊ g��ϡ��Ũ����ʱ�����ɽ�ˮ����Ũ������������ԭ��
��3����ʯ����һ����Ҫ�ļ�ڹ�ũ�������ճ������ж���ʮ�ֹ㷺��Ӧ�ã���ҵ���������ô���ʯ����Ҫ�ɷ�̼��ƣ�������������ȡ��ʯ�ң�Ȼ��������ʯ����ˮ��Ӧ����ȡ��ʯ�ң�����д���йط�Ӧ�Ļ�ѧ����ʽ��
��4��Ϊ̽��һƿ�������ƹ���ı��������ͬѧ�ǽ���������ʵ�飮
��ȡ�����ù�����Ʒ�����Թ��У������м���һ����ɫ��Һ�����������ݲ�����˵������Ʒ�к���̼���ƣ��ɴ˿�ȷ���ù����ѷ������ʣ�����ɫ��Һ������ ��
��Ϊ̽���ù������Ƿ���δ���ʵ��������ƣ�ͬѧ���ֽ��������±���ʾ��ʵ�飮��֪̼���Ƶ�ˮ��Һ�ʼ��ԣ����Ĵ��ڻ���������Ƶļ�����ɸ��ţ������ͼ�в������ʵ��ܽ��Ա���20�棩���ṩ����Ϣ�����±���д������
��5����73 g��������Ϊ20%��������127g����������Һǡ����ȫ�кͣ��Լ��㷴Ӧ��������Һ�����ʵ�����������
��1��ͬѧ�Ǵ�ʢŨ�����Ũ�����Լ�ƿ��ƿ�ǣ��������ܰ��������ֿ���������Ϊʲô��
��2����ͼ��ij�Լ�ƿ��ǩ�ϵ����ݣ�Ҫ��30 g����Ũ����ϡ��Ϊ40%�����ᣬ��Ҫˮ������Ϊ
| Ũ���ᣨ�������� ��ѧʽ��H2SO4 ��Է���������98 �ܶȣ�1.84g/cm3 ����������98% |
��4��Ϊ̽��һƿ�������ƹ���ı��������ͬѧ�ǽ���������ʵ�飮
��ȡ�����ù�����Ʒ�����Թ��У������м���һ����ɫ��Һ�����������ݲ�����˵������Ʒ�к���̼���ƣ��ɴ˿�ȷ���ù����ѷ������ʣ�����ɫ��Һ������
��Ϊ̽���ù������Ƿ���δ���ʵ��������ƣ�ͬѧ���ֽ��������±���ʾ��ʵ�飮��֪̼���Ƶ�ˮ��Һ�ʼ��ԣ����Ĵ��ڻ���������Ƶļ�����ɸ��ţ������ͼ�в������ʵ��ܽ��Ա���20�棩���ṩ����Ϣ�����±���д������
| ������\������ | OH- | NO3- | Cl- | SO42- | CO32- |
| H+ | �ܡ��� | �ܡ��� | �� | �ܡ��� | |
| Na+ | �� | �� | �� | �� | �� |
| Ba2+ | �� | �� | �� | ���� | ���� |
| ʵ��Ŀ�� | ʵ����� | ���� | ���ۻ�ѧ����ʽ |
��ȥ̼���� |
ȡ�����ù�����Ʒ����ˮ�����Һ���μ������� ��ַ�Ӧ����� |
�а�ɫ�������� |
�йط�Ӧ�Ļ�ѧ����ʽΪ |
| �����Ƿ����������� | ����Һ�еμӷ�̪��Һ | ����Ʒ�к����������� |
 18���ᡢ������й㷺��;����Ҫ�����
18���ᡢ������й㷺��;����Ҫ�����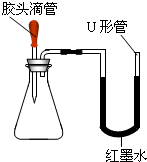 �ᡢ������й㷺��;����Ҫ�����ij��ѧ�С���ͬѧΧ���⼸����������һϵ�е�̽�����
�ᡢ������й㷺��;����Ҫ�����ij��ѧ�С���ͬѧΧ���⼸����������һϵ�е�̽�����