��Ŀ����
����Ŀ����Ӱ�����˵����У������ȼ�����������������ķ������������������������������ij�����ɹ��ѵ�������Σ�չ����С����̽���������ڿ����еı�ը�����������˽��ȼ�����嶼�б�ը���ޣ����ڱ�ը����ʱ����ըҲ���Ż𣬸��ڱ�ը����ʱ���ᱬը��ȼ�ա�������ʦָ������Ʋ����������ʵ�飺
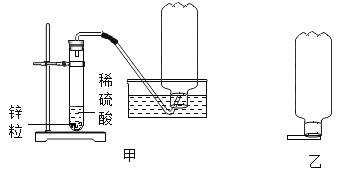
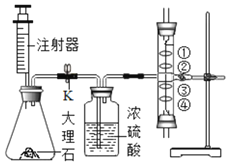
����1������ʵ������ȡ�������Ƶ�װ�ã���ͼ�ף��ÿ�Ȫˮƿ�ռ���������ֱ���5%��100%����������ƿ��
����2����ƿ�ӵ��ۺ��ƿ�������ڻ���ϣ���ͼ�ң�����һ������ȼ������ʵ�����������
H2�������/% | 5 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
��ȼʱ������ | ��ȼ���� | �� ��ը | ǿ ��ը | ǿ ��ը | ǿ ��ը | ǿ ��ը | ǿ ��ը | �� ��ը | ����ȼ�� | ����ȼ�� |
��1������1���ռ�һƿ�������Ϊ50%�����ķ�����______��
��2������ʵ�����������ڿ����еı�ը������______��
��3��С������������۵ķ�Χ�����Ը��Ӿ�ȷ����һ��������______��
���𰸡���ƿ������װһ��ˮ��Ȼ������ˮ����ˮ�ų� 10%-70% ȡ�������������5%-10%��70%-80%������1%Ϊ�ݶ�����ȼʵ�飬�۲��Ƿ�ᱬը
��������
��1���ռ�һƿ�������Ϊ50%�����ķ�������ƿ������װһ��ˮ��Ȼ������ˮ����ˮ�ų��������ƿ������װһ��ˮ��Ȼ������ˮ����ˮ�ų���
��2������ʵ�����������ݿ�֪�������ڿ����еı�ը������10%-70%�����10%-70%��
��3��������ը��Χ�ⶨ�ĸ���ȷ���ɽ������²���ȡ�������������5%-10%��70%-80%������1%Ϊ�ݶ�����ȼʵ�飬�۲��Ƿ�ᱬը�����ȡ�������������5%-10%��70%-80%������1%Ϊ�ݶ�����ȼʵ�飬�۲��Ƿ�ᱬը��

 ��У����ϵ�д�
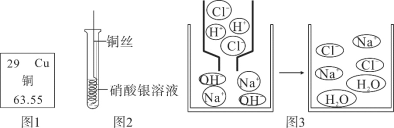
��У����ϵ�д�����Ŀ����ѧϰ��ȼ����������Ŀ����ϣ���ʦ����һ����Ȥ��ʵ�飺��һ��ͭ˿�Ƴ���Ȧ���������������(��ͼ��ʾ)���۲쵽��������Ϩ��
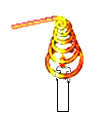
(1)̽��һ������Ϩ���ԭ��
��������裺a��ͭ��Ȧ�����˿�ȼ�b��ͭ��Ȧ�����˿�����c��ͭ��Ȧ���ȣ������������Ż�����¡�
�����뽻�������۲����ͬѧ�Ƿ��˲���a��b��������___��
��Ʋ���������ʵ�飺
ʵ����� | ʵ������ | ���� |
��ͭȦ���Ⱥ�___ | ___ | ����c��ȷ |
(2)̽�����������������ʵ���ҷ���ͭ˿�����ں�ɫ������ʲô�أ�
��������裺��̿�ڣ���___����̿�ں�����ͭ��
���������ϣ�������ͭ����ϡ���ᷴӦ��������ͭ(��Һ����ɫ)��ˮ��̿�ڲ���ϡ���ᷴӦ��
���۽�����ͭ˿���������̿�ڻ�����ͭ��ԭ����___(дһ������)��
ʵ����� | ʵ������ | ���� |
ȡ������ɫ�������Թ��У�___ | ___ | �������ȷ |