��Ŀ����
����Ŀ����֪ij������θ��ҩƷ����Ҫ�ɷ�����̼��þƬ����ѧʽΪAlaMgb��OH��16CO34H2O������ҩƷ�е������ɷ����Ȳ��ֽ⡢������ˮ�Ҳ���ϡ���ᷴӦ��ij�о���ѧϰС�����������ʵ��̽����̼��þƬ����ɡ�
�������ϣ�
���Ȼ�����һ�ְ�ɫ��״���ʪ�Լ�ǿ����¶�ڿ����м��׳��⣻
��ʯ����CaO��NaOH�Ĺ������
��ʵ��һ��ȡm g��ҩƬ���гɷ�ĩ����Ӳ�ʲ������У����ȡ�
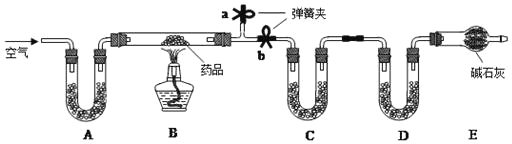
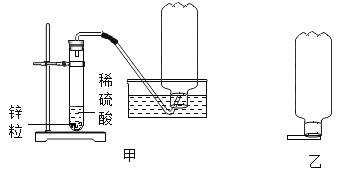
��1����̼��þƬ���ȷֽ�IJ���ΪAl2O3��MgO��CO2��ˮ����Ҫ�ⶨ���ɵ�CO2��ˮ��������װ��C��D ��ʢ�ŵ�ҩƷӦѡ��C_____��D_____��������ţ�
��Ũ���� ����ʯ�� ���Ȼ��� ������
��2��ʵ�鿪ʼǰ�رյ��ɼ�_____����a��b��������һ�����ɼУ���ͨ��һ��ʱ�������װ��A��ʢ�ŵ��Ǽ�ʯ�ң�����������_____��
��3��һ��ʱ��ر���Ӧ�ĵ��ɼУ���ȼ�ƾ��Ƽ��ȣ���ַ�Ӧ��ֹͣ���ȣ�����ͨ��һ��ʱ�������Ŀ����_____��
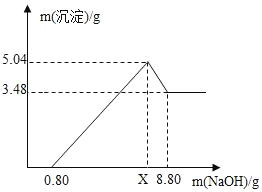
��ʵ�������ȡm g��ҩƬ������100 g9.8%��ϡ���ᣬ��ַ�Ӧ����ȥ������õ�����MgSO4��Al2��SO4��3�����ʵ���Һ������Һ������������ϡNaOH��Һ�����������������������Һ��NaOH�������Ĺ�ϵ��ͼ��
��֪��MgSO4 +2NaOH��Mg��OH��2��+Na2SO4
Al2��SO4��3 +6NaOH��2Al��OH��3��+3Na2SO4
Al��OH��3 +NaOH��NaAlO2 +2H2O
Mg��OH��2������NaOH��NaAlO2������ˮ��
��4��ͼ�е�������Һ��NaOH������Ϊ0.80gʱ��û�г�����˵����Һ�к���_____����������Һ��NaOH��������xʱ�����ɵij�����_____������Al��OH��3��������_____g��x��_____g����д��X�ļ�����̣�
��5��ҽ��������ø�ҩƬʱһ��Ҫ��������̷�����������ѧ��֪ʶ�������������������_____��
��6����ȷ����̼��þ�Ļ�ѧʽΪ_____��
���𰸡��� �� b ��ȥ�����еĶ�����̼��ˮ��������ֹ��ʵ�����IJⶨ��ɸ��� ʹ���ɵ�ˮ�Ͷ�����̼��ȫ��C��D װ������ H2SO4 Mg(OH)2��Al(OH)3 1.56 8 ���ڳ������ Al2Mg6(OH)16CO3��4H2O
��������
ʵ��һ
��1�����������ʵ��װ�ÿ�֪����Ʒ��̼��þƬ�ڼ��������·ֽ���������þ�������CO2��H2O��װ��C��Ӧ��������ˮ�����ʣ����������ն�����̼���壬������ȷ�����ɵ�ˮ�Ͷ�����̼���Ե�����������װ��C��������ˮ������������ˮCaCl2�������գ�װ��D�����ն�����̼�����װ�ã��������ü�ʯ�����ն�����̼���壬�ʴ�Ϊ���ۣ� �ڣ�
��2��ʵ�鿪ʼǰ�رյ��ɼ�b�����ɼ�a����ͨ��һ��ʱ���������Ŀ���ǸϾ�װ��B�еĶ�����̼��ˮ������ȷ��CD��װ�����յ�ˮ������������̼ȫ������Ʒ�ֽ����ɵģ�
��3����B���ľƾ��Ƶ�ȼ���Լ���Ӧ������һ��ʱ������ȻҪͨ�������Ŀ����ʹ���ɵ�ˮ�Ͷ�����̼��ȫ��C��D װ�����գ�
ʵ�����
��4����ͼ�������NaOH����0.80g����û�г�����˵����Һ�����ᣬ������NaOH��������xʱ�����ɵij�����������þ������������ͼ���������3.48gΪMg��OH��2��Al��OH��3��������Ϊ5.04-3.48g=1.56g��
��������þ��Ӧ���������Ƶ�����Ϊx������������Ӧ���������Ƶ�����Ϊy��
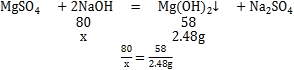
��x=4.8g
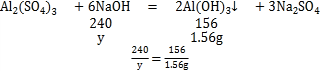
y=2.4g
��x=4.8g+2.4g+0.8g=8g��
��5��ҽ��������ø�ҩƬʱһ��Ҫ��������̷�����������ѧ��֪ʶ����������������� �ǣ����ڳ�����գ�
��6�����������غ㶨�ɣ���Ӧǰ��Ԫ�ص��������䣬�����������ݣ�3.48gMg��OH��2��þԪ�ص�����Ϊ1.44g��ԭ�Ӹ���Ϊ6����Al��OH��3��������1.56g����Ԫ�ص�����Ϊ0.54g��ԭ�Ӹ���Ϊ2��������̼��þ�Ļ�ѧʽΪAl2Mg6��OH��16CO34H2O��

 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�����Ŀ��ij��ȤС��ͬѧ������ͼ��ʾװ�ý��С���������ͭ��Һ��Ӧ����ʵ��ʱ�����ַ�Ӧ��ʼ���Թ��г��ֺ�ɫ����ͬʱ������ɫ��ζ���塣��ȤС��ͬѧ����������̽����

ʵ��1����5����ͬ���������ͭ��Һ�зֱ������澭����ĥ��������ͬ���ȵ��������������£�
ʵ����� | �� | �� | �� | �� | �� | �� | �� |
����ͭ��ҺŨ�� | 24% | 12% | 8% | 6% | 5% | 3% | 1% |
������� | �ܿ� | �� | �Ͽ� | �� | �Ͽ� | �� | �� |
ʵ��2������������Ϊ8%������ͭ��Һ�ֱ������澭����ĥ��������ͬ���ȵ��������������£�
ʵ����� | �� | �� | �� | �� | �� |
ˮԡ�¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 |
������� | �Ͽ� | �� | �ܿ� | �ܿ� | �ܿ� |
��1��ʵ��1��Ŀ����______��
��2��ʵ��2�Ľ�����______��
��3������ʵ��1��ʵ��2���Ʋ�50�� 5% ����ͭ��Һ��������Ӧ��������________��
����˼�����ۣ�
��4��ʵ��2û�н����¶�Ϊ70���ʵ�飬������_______��
��5��������IJ��룬�����е�������______��
����Ŀ����Ӱ�����˵����У������ȼ�����������������ķ������������������������������ij�����ɹ��ѵ�������Σ�չ����С����̽���������ڿ����еı�ը�����������˽��ȼ�����嶼�б�ը���ޣ����ڱ�ը����ʱ����ըҲ���Ż𣬸��ڱ�ը����ʱ���ᱬը��ȼ�ա�������ʦָ������Ʋ����������ʵ�飺
����1������ʵ������ȡ�������Ƶ�װ�ã���ͼ�ף��ÿ�Ȫˮƿ�ռ���������ֱ���5%��100%����������ƿ��
����2����ƿ�ӵ��ۺ��ƿ�������ڻ���ϣ���ͼ�ң�����һ������ȼ������ʵ�����������
H2�������/% | 5 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
��ȼʱ������ | ��ȼ���� | �� ��ը | ǿ ��ը | ǿ ��ը | ǿ ��ը | ǿ ��ը | ǿ ��ը | �� ��ը | ����ȼ�� | ����ȼ�� |
��1������1���ռ�һƿ�������Ϊ50%�����ķ�����______��
��2������ʵ�����������ڿ����еı�ը������______��
��3��С������������۵ķ�Χ�����Ը��Ӿ�ȷ����һ��������______��