��Ŀ����
��2011�꽭���Ͼ���27�⣩ij��ȤС��Ļ�ѧ̽��Ũ����������(̼�ظ�)��Ӧ���������ijɷ֡�
���������ϡ��٣ƣ���Ũ�����ӣ��� ��Ӧ�����ȣ��Уӣ������� ��
�ڣ���Ũ�����ӣ��� ��Ӧ�����ȣ��Уã����ͣӣ������ɣ�
�ۣӣ��� ��ʹ����ʯ��ˮ����ǣ�
�ܣӣ�����ʹ�ں���Һ�ĺ�ɫ��ȥ�����ã������ܡ�
�ݣӣ�����ʹ���Ը��������Һ��ɫ�����ã������ܡ�
��ʯ�ҵ���Ҫ�ɷ��ǣΣ�ϣȺͣã��
��ʵ��̽������ȡ24.0����������60.0����Ũ�����У����ȣ���ַ�Ӧ��õ���ҺX���ռ�������Y����ͬѧͨ��ʵ��ⶨ����֪����Y�Уӣ������������Ϊ66.7%��ͬѧ����Ϊ����Y�л����ܺ��У�����Z���壬��Z��������� ��̽��ʵ�����Ҫװ������ͼ��ʾ��

��װ��A���Լ��������� ��
��Ϊȷ��Z�Ĵ��ڣ��轫װ�ü�(��ͼ��ʾ)��������װ��(����) ֮�䡣װ�ü��е�Һ�������� ��

����ʵ���У�����۲쵽װ��D�� ����װ��E�� �����ȷ��Y�к��У������塣
�������Ҫ�ⶨ���������Y�У����ĺ�����(����ԼΪ0.01��)�������ò���
��������ķ����⣬ (ѡ��ܡ����ܡ�)�ó�����ͼװ��Dװ��E�������仯�ķ�����ͨ�����㲢˵�����жϵ����ɡ�
���������ϡ��٣ƣ���Ũ�����ӣ��� ��Ӧ�����ȣ��Уӣ������� ��
�ڣ���Ũ�����ӣ��� ��Ӧ�����ȣ��Уã����ͣӣ������ɣ�
�ۣӣ��� ��ʹ����ʯ��ˮ����ǣ�
�ܣӣ�����ʹ�ں���Һ�ĺ�ɫ��ȥ�����ã������ܡ�
�ݣӣ�����ʹ���Ը��������Һ��ɫ�����ã������ܡ�
��ʯ�ҵ���Ҫ�ɷ��ǣΣ�ϣȺͣã��
��ʵ��̽������ȡ24.0����������60.0����Ũ�����У����ȣ���ַ�Ӧ��õ���ҺX���ռ�������Y����ͬѧͨ��ʵ��ⶨ����֪����Y�Уӣ������������Ϊ66.7%��ͬѧ����Ϊ����Y�л����ܺ��У�����Z���壬��Z��������� ��̽��ʵ�����Ҫװ������ͼ��ʾ��

��װ��A���Լ��������� ��
��Ϊȷ��Z�Ĵ��ڣ��轫װ�ü�(��ͼ��ʾ)��������װ��(����) ֮�䡣װ�ü��е�Һ�������� ��

����ʵ���У�����۲쵽װ��D�� ����װ��E�� �����ȷ��Y�к��У������塣
�������Ҫ�ⶨ���������Y�У����ĺ�����(����ԼΪ0.01��)�������ò���
��������ķ����⣬ (ѡ��ܡ����ܡ�)�ó�����ͼװ��Dװ��E�������仯�ķ�����ͨ�����㲢˵�����жϵ����ɡ�
��CO2 �� ��ȥSO2 �� BC ����ʯ��ˮ �� ��ɫ������ֺ�ɫ ��ɫ���������ɫ ����(����)(�ش��ܡ����ܡ�ֻҪ����ƥ����ɸ���)
�⣺��0.01��������D������ͭ��ַ�Ӧ������ˮ������ΪX��
H2+CuO Cu+H2O
Cu+H2O
2 18
0.01�� X
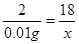
X��0.09g
������ˮ��������֪��
ˮ�к��е���Ԫ�ص�����Ϊ��0.09��-0.01��=0.08�ˣ���μӷ�Ӧ������ͭ����Ԫ�ص�����Ϊ0.08�ˡ�
��Ӧǰ��װ��D�������ı仯Ϊ�μӷ�Ӧ������ͭ����Ԫ�ص�������װ��E�������ı仯Ϊ����ˮ��������װ��D��װ��E�������仯�ֱ�Ϊ0.08�˺�0.09�ˡ�
�ܣ����ܵ�����ƽ������װ��D��װ��E�IJ�����
(���ܣ���Ϊʵ������������ƽֻ��ȷ�Ƶ�0.1��)
(�����еļ����粻ͨ����ѧ����ʽ���У����̺������ɸ���
�⣺��0.01��������D������ͭ��ַ�Ӧ������ˮ������ΪX��
H2+CuO
 Cu+H2O
Cu+H2O2 18
0.01�� X
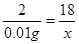
X��0.09g
������ˮ��������֪��
ˮ�к��е���Ԫ�ص�����Ϊ��0.09��-0.01��=0.08�ˣ���μӷ�Ӧ������ͭ����Ԫ�ص�����Ϊ0.08�ˡ�
��Ӧǰ��װ��D�������ı仯Ϊ�μӷ�Ӧ������ͭ����Ԫ�ص�������װ��E�������ı仯Ϊ����ˮ��������װ��D��װ��E�������仯�ֱ�Ϊ0.08�˺�0.09�ˡ�
�ܣ����ܵ�����ƽ������װ��D��װ��E�IJ�����
(���ܣ���Ϊʵ������������ƽֻ��ȷ�Ƶ�0.1��)
(�����еļ����粻ͨ����ѧ����ʽ���У����̺������ɸ���
��������1������̼����Ũ���ᷴӦ���з�����
��2������SO2��������KMnO4��Һ��Ӧ���з�����
��3��������֤������̼����ʯ��ˮ��֤����ʯ�������ն�����̼�Ľ��з�����
��4�������������л�ԭ�ԣ�����ˮ���з�����
��5������������ԭ����ͭд������ʽ���г�����ʽ���н��
��𣺽⣺��1��C��ŨH2SO4�ڼ���ʱ���Ȼ�����CO2�������к���̼Ԫ�أ����ԣ��ʴ�Ϊ��CO2��
��2��������ؾ���ǿ�����ԣ������������Ӧ�������������̼��Ӧ�����������Զ�����̼�ļ���������ţ��ʴ�Ϊ����ȥSO2��
��3�����������̼��Ҫ��ʯ��ˮ���飬C�еļ�ʯ����Ϊ�˳�ȥ������̼���ʴ�Ϊ��B C������ʯ��ˮ��
��4���������л�ԭ�ԻὫ��ɫ������ͭ��ԭ�ɺ�ɫ��ͭ�����ɵ�ˮ��ʹ��ˮ����ͭ����ɫ���ʴ�Ϊ����ɫ������ֺ�ɫ����ɫ���������ɫ��
��5���������е������г�����ʽ��Ȼ����ݱ���ʽ���ˮ��������Ȼ��������е����ݱȽϽ��
�ܣ����ܣ�
�⣺��0.01g��H2��D�е�CuO��ַ�Ӧ������ɵ�ˮ������Ϊx
H2+CuO Cu+H2O
Cu+H2O
2 18
0.01g x
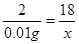
x=0.09g
�����ɵ�ˮ��������֪��
ˮ�к��е���Ԫ������Ϊ0.09g-0.01g=0.08g����μӷ�Ӧ��CuO����Ԫ�ص�����Ϊ0.08g��
��Ӧǰ��װ��D�е������仯Ϊ�μӷ�Ӧ��CuO����Ԫ�ص�������װ��E�������仯Ϊ����ˮ��������װ��D��װ��E�������仯�ֱ�Ϊ0.08g��0.09g��
�ܣ����õ�����ƽ������װ��D��װ��E�IJ�����
�����ܣ���Ϊʵ������������ƽֻ�ܳ�0.1g����
��2������SO2��������KMnO4��Һ��Ӧ���з�����
��3��������֤������̼����ʯ��ˮ��֤����ʯ�������ն�����̼�Ľ��з�����
��4�������������л�ԭ�ԣ�����ˮ���з�����
��5������������ԭ����ͭд������ʽ���г�����ʽ���н��
��𣺽⣺��1��C��ŨH2SO4�ڼ���ʱ���Ȼ�����CO2�������к���̼Ԫ�أ����ԣ��ʴ�Ϊ��CO2��
��2��������ؾ���ǿ�����ԣ������������Ӧ�������������̼��Ӧ�����������Զ�����̼�ļ���������ţ��ʴ�Ϊ����ȥSO2��
��3�����������̼��Ҫ��ʯ��ˮ���飬C�еļ�ʯ����Ϊ�˳�ȥ������̼���ʴ�Ϊ��B C������ʯ��ˮ��
��4���������л�ԭ�ԻὫ��ɫ������ͭ��ԭ�ɺ�ɫ��ͭ�����ɵ�ˮ��ʹ��ˮ����ͭ����ɫ���ʴ�Ϊ����ɫ������ֺ�ɫ����ɫ���������ɫ��
��5���������е������г�����ʽ��Ȼ����ݱ���ʽ���ˮ��������Ȼ��������е����ݱȽϽ��
�ܣ����ܣ�
�⣺��0.01g��H2��D�е�CuO��ַ�Ӧ������ɵ�ˮ������Ϊx
H2+CuO
 Cu+H2O
Cu+H2O2 18
0.01g x
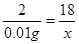
x=0.09g
�����ɵ�ˮ��������֪��
ˮ�к��е���Ԫ������Ϊ0.09g-0.01g=0.08g����μӷ�Ӧ��CuO����Ԫ�ص�����Ϊ0.08g��
��Ӧǰ��װ��D�е������仯Ϊ�μӷ�Ӧ��CuO����Ԫ�ص�������װ��E�������仯Ϊ����ˮ��������װ��D��װ��E�������仯�ֱ�Ϊ0.08g��0.09g��
�ܣ����õ�����ƽ������װ��D��װ��E�IJ�����
�����ܣ���Ϊʵ������������ƽֻ�ܳ�0.1g����

��ϰ��ϵ�д�
�����Ŀ


 a)g
a)g