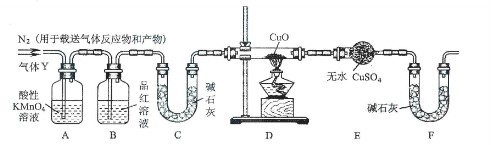
��Ŀ����
С�ִӺ���ʰ��һЩǶ��ɳ���ı��ǣ���Ҫ�ɷ���̼��ƣ���Ϊ�ⶨ����̼��Ƶĺ���������������ʵ�飺���ȳ�ȡ50g���ǣ�Ȼ��150mlϡ�����5�μ��루������Ʒ�е����ʲ���ϡ���ᷴӦ��Ҳ������ˮ����ʵ������е����ݼ�¼���±���
��1��x=__________��
��2��С�ֲ�ñ�����Ʒ��̼��Ƶ���������Ϊ__________��
��3���������ɵĶ�����̼ȫ���ռ����������ж��ٿˣ�д��������̣���
| ϡ��������/ml | ��һ�� 30 | �ڶ��� 30 | ������ 30 | ���Ĵ� 30 | ����� 30 |
| ʣ����������/g | 40 | x | 20 | 10 | 5 |
��2��С�ֲ�ñ�����Ʒ��̼��Ƶ���������Ϊ__________��
��3���������ɵĶ�����̼ȫ���ռ����������ж��ٿˣ�д��������̣���
��1��x=30����2��90%��
��3��19.8g
��3��19.8g
���⿼����Ǹ��ݻ�ѧ��Ӧ����ʽ�ļ��㡣
��1������ʵ������е����ݼ�¼����һ�μ���ϡ����30mlʱ��ʣ����������40g������жϷ�Ӧ��̼���10g����ϵ����κ͵��ĴΣ����ƶ�ÿ�μ���30mlϡ�����Ӧ��̼���10g����x=30
��2���ɵ���μ���ϡ����30mlʱ��ʣ���������5g�����жϴ�ʱ̼�������ȫ��Ӧ��ʣ�����Ϊ��Ʒ�е����ʣ��ɴ˿ɼ���ʯ��ʯ��Ʒ��̼��Ƶ�������������50g��5g��/50g��100�G=��90%��
��3���跴Ӧ�в����Ķ�����̼��������Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
45g x
100��44 =45g��x
x=19.8g
�𣺷�Ӧ�в����Ķ�����̼��������Ϊ19.8g
��1������ʵ������е����ݼ�¼����һ�μ���ϡ����30mlʱ��ʣ����������40g������жϷ�Ӧ��̼���10g����ϵ����κ͵��ĴΣ����ƶ�ÿ�μ���30mlϡ�����Ӧ��̼���10g����x=30
��2���ɵ���μ���ϡ����30mlʱ��ʣ���������5g�����жϴ�ʱ̼�������ȫ��Ӧ��ʣ�����Ϊ��Ʒ�е����ʣ��ɴ˿ɼ���ʯ��ʯ��Ʒ��̼��Ƶ�������������50g��5g��/50g��100�G=��90%��
��3���跴Ӧ�в����Ķ�����̼��������Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
45g x
100��44 =45g��x
x=19.8g
�𣺷�Ӧ�в����Ķ�����̼��������Ϊ19.8g

��ϰ��ϵ�д�
�����Ŀ
 Si+ 4HCl�������Ҫ���14g�裨Si����������Ҫ�������ٿˣ�
Si+ 4HCl�������Ҫ���14g�裨Si����������Ҫ�������ٿˣ� 2Na+3N2������������23g����ͬʱ���������������Ƕ��٣�
2Na+3N2������������23g����ͬʱ���������������Ƕ��٣� 2NaOH��H2
2NaOH��H2 ��Cl2
��Cl2