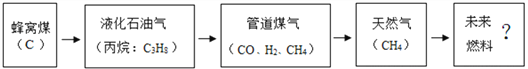
��Ŀ����
����Ŀ��Ǧ������������������ʹ�ù㷺���乹��ʾ��ͼ��ͼ1���ش��������⣺
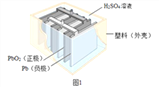
(1)ͼ1Ǧ���طŵ�����У���Ӧ�Ļ�ѧ����ʽΪ Pb+PbO2+2H2SO4�T2PbSO4+2H2O���ݴ˿�֪��Ǧ�����ڷŵ�ʱ����Һ��pH����_____(����������������С������������)��
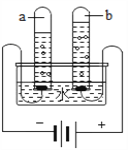
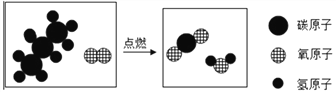
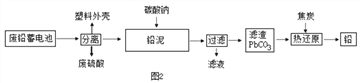
(2)��ͼ2�ǻ��շ�Ǧ���ص�һ�ֹ������̡�
��д����̿�Ȼ�ԭ����Ǧ�Ļ�ѧ����ʽ�� ________________________
�ڽ�����ͼ�еķ��������Һ��һ��������ϣ��ٽ����õĻ��Һ��____�����½ᾧ�����˵Ȳ�������������Na2SO4��10H2O���塣
�������һЩ�����۵�����ݣ�
���� | �� | Ǧ | �� | �� |
�۵��M�� | 231.9 | 327.5 | 271.3 | 320.9 |
�ճ����ñ���˿���顢Ǧ�������ӵȽ�����ɣ����۵�ԼΪ________________��
A�� 231.9-327.5�� B��271.3-320.9�� C��60-80�� D��20-40��
�ܷ�����ֱ���ŷŻ���Ⱦ��������ѡ����������кͺ����ŷţ�
���� | CaCO3 | NH3 | Ca(OH)2 |
�г��ο���(Ԫ/kg ) | 1.8 | 6.5 | 2.0 |
��֪��2NH3+H2SO4�T(NH4)2SO4�����Ҫ�����ٵ�Ǯ���к͵���������Ũ�ȵķ����ᣬ��Ӧѡ��_________ ��
���𰸡� �� �� ����Ũ�� C Ca(OH)2
��������(1)Ǧ���طŵ�����У���Ӧ�Ļ�ѧ����ʽΪ Pb+PbO2+2H2SO4�T2PbSO4+2H2O����֪��Ǧ�����ڷŵ�ʱ���������٣���Һ�����Ա�������Һ��pH����������(2)�ٸ���������̼��Ǧ�ֽ���������Ǧ�Ͷ�����̼����̿������Ǧ��Ӧ����Ǧ�Ͷ�����̼����ѧ����ʽ�� 2PbO + C ![]() 2Pb+CO2���� �ڽ�����ͼ�еķ��������Һ��һ��������ϣ��ٽ����õĻ��Һ������Ũ�������½ᾧ�����˵Ȳ�������������Na2SO4��10H2O���壻�۱���˿���۵㲻��̫�ߣ��������������ã�����̫�������۶ϣ���Ϊ60~80����ã���4��
2Pb+CO2���� �ڽ�����ͼ�еķ��������Һ��һ��������ϣ��ٽ����õĻ��Һ������Ũ�������½ᾧ�����˵Ȳ�������������Na2SO4��10H2O���壻�۱���˿���۵㲻��̫�ߣ��������������ã�����̫�������۶ϣ���Ϊ60~80����ã���4��
CaCO3+H2SO4�TCaSO4+H2O+CO2��
10098
Ca(OH)2+H2SO4�TCaSO4+2H2O
7498
2NH3+H2SO4�T(NH4)2SO4
3498
2NaOH+H2SO4�TNa2SO4+2H2O
8098
���紦���������������98Kg������Ҫ̼��Ƶ�Ǯ��Ϊ��100Kg��1.8Ԫ/Kg=180Ԫ����Ҫ�������Ƶ�Ǯ��Ϊ��74Kg��2.0Ԫ/Kg=148Ԫ����Ҫ������Ǯ��Ϊ��34Kg��6.5Ԫ/Kg=221Ԫ����Ҫ�������Ƶ�Ǯ��Ϊ��80Kg��11.5Ԫ/Kg=920Ԫ��ʹ�������������õ�Ǯ�����٣�ѡ����C��