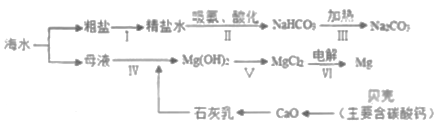
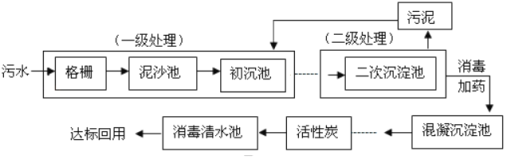
��Ŀ����
����Ŀ����ʦ�ڳ�������ʱ�����Ϻ��ټӴף����ò��ȱ�������ɿڣ�����ʵ������һ����ѧ��С���ܣ����е��������Ͼ��е��Ҵ���Ӧ����������ζ�������������±����Ǽ��ֳ���������������������⣺
�������� | ������� | �������� | ������� | ���ᶡ�� |
��ѧʽ | C2H4O2 | C4H8O2 | C6H12O2 | X |
��1�����������̼Ԫ�ء���Ԫ�ء���Ԫ�ص�������Ϊ_____��������Ԫ�ص���������Ϊ_____��������0.1%��
��2���ȽϹ�����ѧϰ����Ҫ���������ݱ��Ʋ�X�Ļ�ѧʽ��_____��
��3�������������Ԫ�ص���������_____����������������С�������������������ᶡ������Ԫ�ص�����������
��4���������е����������������ڴ�лʱ���е�̼Ԫ����ȫ��ת��ΪCO2����44mg������������ȫ��л������CO2������Ϊ���٣�����д��������̣�_________
���𰸡�6��1��8 6.7% C8H16O2 ���� 88mg
��������
��1�����������̼Ԫ�ء���Ԫ�ء���Ԫ�ص�������Ϊ��12��2������1��4������16��2����24��4��32��6��1��8��������Ԫ�ص���������Ϊ![]() ��6.7%�����6��1��8��6.7%��
��6.7%�����6��1��8��6.7%��
��2�����ݱ����ṩ�����ʵĻ�ѧʽ���Կ�������֬�����ʸ�һ�������У�����ָ̼ԭ�ӵĸ���Ϊ1������ָ̼ԭ�ӵĸ���Ϊ2������ָ̼ԭ�ӵĸ���Ϊ3������ָ̼ԭ�ӵĸ���Ϊ4������ԭ����Ŀ��̼ԭ�Ӹ�����2������ԭ�ӵĸ���Ϊ2�����Ʋ�X�Ļ�ѧʽ��C8H16O2�����C8H16O2��
��3�������������Է�������С�ڶ��ᶡ����ÿ�������ж���������ԭ�ӣ�����Ԫ�ص������������ڶ��ᶡ������Ԫ�ص�����������������ڣ�
��4������̼Ԫ���غ㣬���������Ͷ�����̼��������ϵ�����У�C4H8O2��4CO2��������Ķ�����̼������Ϊx�����У�
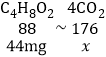
![]()
![]()
x��88mg
��44mg������������ȫ��л������CO2������Ϊ88mg��

 ����������ϵ�д�
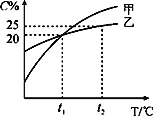
����������ϵ�д�����Ŀ��Fenton�������ڴ������ѽ����л���Ĺ�ҵ��ˮ����ԭ��������Fe2+��H2O2��Ӧ�����ܽ�����Ⱦ��Ļ��Գɷ֡������ø÷��������л���Ⱦ��p-CP��̽���й����ضԽ��ⷴӦ���ʵ�Ӱ�졣ʵ��ʱ��p-CP�ij�ʼŨ����ͬ���¶Ⱥ�Ϊ25���40�森ʵ����p-CP��Ũ����ʱ��仯�Ĺ�ϵ��ͼ��
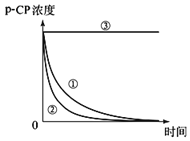
��ش�
��1�����������ʵ����Ʊ���
ʵ�� ��� | ʵ��Ŀ�� | �¶�/�� | pH | Ũ��/��mgL-1�� | |
H2O2 | Fe2+ | ||||
�� | Ϊ�ڢ��ṩ���� | 25 | 3 | 204 | 16.8 |
�� | ̽���¶ȶԽ��ⷴӦ���ʵ�Ӱ�� | ______ | 3 | 204 | 16.8 |
�� | ̽����ҺpH�Խ��ⷴӦ���ʵ�Ӱ�� | 25 | 10 | 204 | 16.8 |
��2��ʵ��١��ڱ����¶����ߣ����ⷴӦ����______��
��3����һ��ʵ�鷢�֣��¶ȹ���ʱ�����ⷴӦ���ѷ�������������Լ�H2O2�ĽǶȷ���ԭ��______��
��4��Ϊ�ⶨ��ͬʱ�����л��オ���Ũ�ȣ����ڲ�ͬʱ��ӷ�Ӧ����ȡ������ʹ��ȡ��Ʒ�еĽ��ⷴӦ����ֹͣ������ͼʾ��Ϣ����д��Ѹ��ֹͣ��Ӧ��һ�ַ�����______��