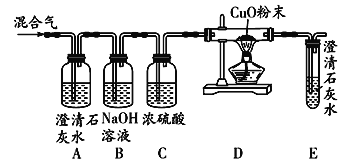
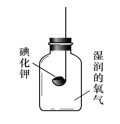
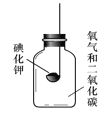
��Ŀ����
����Ŀ����ͼ�dz�����ˮ����������ͼ��
�Ķ�����ͼ���ش��������⣺
��1������ˮ���������У�һ������������ʹ�õĴ���������������_____�仯������������������ѧ�������ù����еĴ���������Ҫ�dz�ȥ_____���ʣ�
��2����������ˮ���г��ö��������������ʶ�ˮ�����������������ȵĻ�ѧʽ��_____��������Ԫ�صĻ��ϼ���_____�ۣ�
��3��Ӳˮ���ճ������ҵ�����г��������㣬��������Ӳˮ�������������ϵͳ��ʯ�ĵò��ʡ������г���_____�ķ�����Ӳˮ����������������Ӳˮ����ˮ��ʹ��_____��
��4������ˮ���������У�����̿��������_____��
��5���������ƣ� Na2FeO4����ˮ����������ʹ�õ�һ�����;�ˮ������ȡ�������ƵĻ�ѧ����ʽ���£�
2Fe��NO3��3+16NaOH+3Cl2��6NaNO3+2Na2FeO4+6X+8H2O����X�Ļ�ѧʽ��_____��
��6����Լ��ˮ��ÿ�����������������ˮ��ʽӦ���ᳫ����_____������ĸ��ţ�
A ϴ�����ԡ¶ʱ����ʱ�ر�ˮ��ͷ
B ����ϵر���ˮ��ϴ��
C ������ˮ��ϴ��ˮ����������
D ����ࡢ�ι�ķ�������ũ�����
���𰸡����� ������ ClO2 +4 ��� ����ˮ �������� NaCl ACD
��������
��1������ˮ���������У�һ������������ʹ�õĴ�������������û���µ��������ɣ����������仯���ù����еĴ���������Ҫ�dz�ȥ���������ʣ�
��2���������ȵĻ�ѧʽ��ClO2�����ݻ������л��ϼ۴�����Ϊ���ԭ����Ԫ�ػ��ϼ�Ϊ-2������Ԫ�صĻ��ϼ���+4��
��3��Ӳˮ���ճ������ҵ�����г��������㣬��������Ӳˮ�������������ϵͳ��ʯ�ĵò��ʡ������г�����еķ�����Ӳˮ����������������Ӳˮ����ˮ��ʹ�÷���ˮ��������ˮ��������ĭ�ٵ���Ӳˮ��������ˮ��������ĭ�������ˮ��
��4������ˮ���������У�����̿���������������á�
��5���������غ㶨�ɷ�Ӧǰ��ԭ�ӵ����༰��Ŀ�����֪����2Fe��NO3��3+16NaOH+3Cl2��6NaNO3+2Na2FeO4+6x+8H2O����X�Ļ�ѧʽ�ǣ�NaCl��
��6��A��ϴ�����ԡ¶ʱ����ʱ�ر�ˮ��ͷ���ܽ�Լ��ˮ�����ᳫ��
B������ϵر���ˮ��ϴ�£����˷�ˮ�������ᳫ��
C��������ˮ��ϴ��ˮ������������һˮ���ã��ܽ�Լ��ˮ�����ᳫ��
D������ࡢ�ι�ķ�������ũ����֣��ܽ�Լ��ˮ�����ᳫ����� ACD��

����Ŀ������֪����������������Һ���Բⶨδ֪��Һ����������������ʵ������һƿ��ǩģ����ϡ���ᣬijͬѧΪ�˲ⶨ��������������������������ʵ�飺
��ȡһ�ྻ����ƿ������Ȼ�������м���һ������ϡ���ᣬ�����뼸����ɫʯ����Һ���������Բ��ƣ������³�����
����������ƿ����μ���������������Ϊ 16%������������Һ���ߵμӱ������۲쵽��Һ�ɺ�ɫǡ�ñ�Ϊ��ɫʱ��ֹͣ�μӣ��ٴγ������������±���
��ƿ | ��ƿ+ϡ���� | ��ƿ+��Ӧ����Һ | |
����/�� | 35.5 | 55.5 | 80.5 |
��1��ʵ�������ĵ�����������Һ����Ϊ______�ˡ�
��2����Ӧ����Һ�е��������������Ƕ���______��
��3������ͬѧ����ͬ����ʵ�飬ȴ�õ���ͬ�Ľ�����þ���pH��ֽ��÷�Ӧ����ƿ����Һ��pHΪ7.8���������������������������ƫ����ƫС�����ƫ���ԭ����______��
����Ŀ����ʦ�ڳ�������ʱ�����Ϻ��ټӴף����ò��ȱ�������ɿڣ�����ʵ������һ����ѧ��С���ܣ����е��������Ͼ��е��Ҵ���Ӧ����������ζ�������������±����Ǽ��ֳ���������������������⣺
�������� | ������� | �������� | ������� | ���ᶡ�� |
��ѧʽ | C2H4O2 | C4H8O2 | C6H12O2 | X |
��1�����������̼Ԫ�ء���Ԫ�ء���Ԫ�ص�������Ϊ_____��������Ԫ�ص���������Ϊ_____��������0.1%��
��2���ȽϹ�����ѧϰ����Ҫ���������ݱ��Ʋ�X�Ļ�ѧʽ��_____��
��3�������������Ԫ�ص���������_____����������������С�������������������ᶡ������Ԫ�ص�����������
��4���������е����������������ڴ�лʱ���е�̼Ԫ����ȫ��ת��ΪCO2����44mg������������ȫ��л������CO2������Ϊ���٣�����д��������̣�_________
����Ŀ��С�����ʵ��̽����п�����ᷴӦ������Ӱ��������ʵ���������
ʵ���� | ������������� | ��������/mL | п | п������/g | 3�����ռ����������/mL |
�� | 20% | 20 | � | 1 | 31.7 |
�� | 20% | 20 | � | 1 | 50.9 |
�� | 30% | 20 | � | 1 | 61.7 |
�� | 30% | 20 | � | 1 | 79.9 |
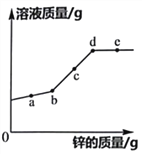
��1��С������ʵ����Ҫ��֤�ļ�����______��
��2��С������ͼװ���ռ����������������������cӦ��______����������a������b����
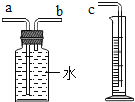
��3�������С��������ʵ�����ϸ���ݣ�
��¼����ʱ�� | ��1����ĩ | ��2����ĩ | ��3����ĩ | ��4����ĩ | ��5����ĩ | ��6����ĩ |
��Ͳ��ˮ�����/mL | 3.1 | 19.8 | 31.7 | 41.3 | 48.9 | 54.9 |
��֪п�����ᷴӦ�Ƿ��ȷ�Ӧ����������������п�����ᷴӦ�����ʱ仯�������������������______��