��Ŀ����
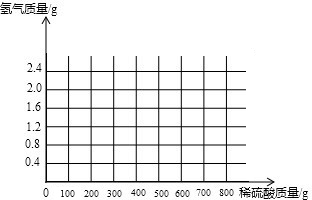
ij��ѧ��ȤС��Ϊ�˲ⶨ���ص�ʯ��ʯ����Ҫ�ɷ���CaCO3��������������������������ʵ�飺ȡ24����Ʒ�����ձ��У�����������100��һ������������ϡ���ᣨ���ʲ�����ˮҲ�����ᷴӦ�����������������������ʣ������������ϵ��ͼ��ʾ�������ͼ�е����ݽ��м��㣺
��1��ʯ��ʯ��Ʒ��CaCO3������Ϊ�����ˣ�
��2��24��ʯ��ʯ��Ʒ�������ַ�Ӧ��������������̼������Ϊ�����ˣ�
��3���������������������������������ݻ�ѧ����ʽд�������ļ��㲽�裩

| ��1������ͼ�������չ��������������� ��2������̼��Ƶ��������÷���ʽ��̼��Ƶ������ɵö�����̼�������� ��3������̼��Ƶ�������ǡ�÷�Ӧʱ���ĵ��Ȼ����������м��㣬��ɵ�������������������� | |
| ��� | �⣺��1����ͼ���֪���շ�Ӧ�����Ĺ���Ϊ4g������������ʣ��IJ���Ӧ�����ʵ�����������ʯ��ʯ��̼��Ƶ�������24g﹣4g=20g�� ��2����ͼ���֪̼���������ǡ�÷�Ӧʱ��������80g�� �⣺��μӷ�Ӧ��������HCl������Ϊx�����ɵĶ�����̼��������y CaCO3+2HCl=CaCl2+H2O+CO2�� 100 73 44 20g x y
x=14.6g y=8.8g ��3����ͼ���֪̼���������ǡ�÷�Ӧʱ��������80g�����������������������Ϊ �ʴ�Ϊ����1��20����2��8.8����3�����������������������Ϊ��18.25%�� |
��

| ��1�� | ��2�� | ��3�� | ��4�� | |
| ����ϡ����������g�� | 200 | 200 | 200 | 200 |
| ʣ�����������g�� | 37.6 | 15.2 | 4 | 4 |
��˵�������������ʲ�����ˮ��Ҳ�������ᷴӦ����
��1����2�β��ʣ���������Ϊ15.2g����ɷ�Ϊ
A���� B������̼ C��̼
��2��������������������Ϊ���٣���д��������̣����������С�����һλ��
��3����������ϡ�����������������Ϊ���٣���д��������̣����������С�����һλ����������Ӧ���ɵ��������������ϡ���������Ĺ�ϵͼ��
| ��Ӧʱ��/�� | 0 | 2 | 4 | 6 | 8 | 10 |
| �ձ�����ʢ��������/�� | 80.0 | 79.0 | 78.3 | 77.9 | 77.8 | 77.8 |
��2����ʯ��ʯ��Ʒ��̼��Ƶ����������Ƕ��٣�

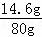 ��100%=18.25%
��100%=18.25% ij��ѧ��ȤС��Ϊ�˴��Բⶨһ��ʯ��ʯ��Ʒ��CaCO3��������������Ʋ���������ʵ�飮ʵ��װ����ͼ��ʾ����ȡ��ϸ��2.60gʯ��ʯ��Ʒ����4�μ���ϡ���ᣬ��ַ�Ӧ�����ٲ�������Ϊֹ����÷�Ӧǰ����й����������
ij��ѧ��ȤС��Ϊ�˴��Բⶨһ��ʯ��ʯ��Ʒ��CaCO3��������������Ʋ���������ʵ�飮ʵ��װ����ͼ��ʾ����ȡ��ϸ��2.60gʯ��ʯ��Ʒ����4�μ���ϡ���ᣬ��ַ�Ӧ�����ٲ�������Ϊֹ����÷�Ӧǰ����й����������