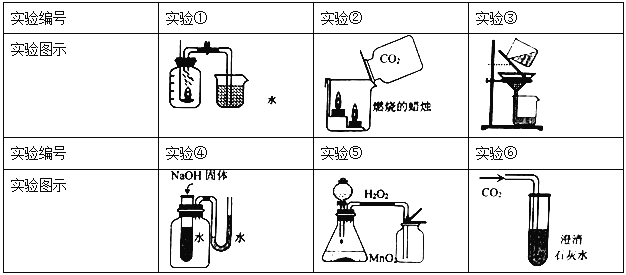
��Ŀ����
����Ŀ��Ϊ�ⶨþ������þ����������þԪ�ص������������ȳ�ȡ10g��������һ�ɾ����ձ��У�Ȼ��ȡ100gij��������������ϡ���ᣬƽ������μ������У���ַ�Ӧ��ʵ�������������±���
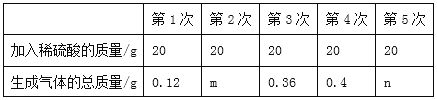
��l���ϱ���m=_____��n=_____��
��2�����������ϡ�����������������_______���������һλС������
��3�����������У�þԪ�ص���������Ϊ_____��
��4������60g������������Ϊ98%��Ũ���ᣬ��������ʵ����������������������ϡ���ᣬ���ˮ_g��
���𰸡� 0.24 0.4 29.4% 58.4% 140g
����������l�����������е�����þ����ϡ���ᷴӦ��þ����ϡ���ᷴӦ��������������ʵ�����ݿ�֪��20gϡ����������þ��Ӧ����0.12g������m=0.36g-0.12g=0.24g�����þ�������㣬��4��ʵ�����������������Ӧ����0.36g+0.12g=0.48g����ʵ������0.4g��˵��þ���㣬���ټ���ϡ����ʱ��Ҳ���ٲ�����������n=![]() 0.4g��
0.4g��
��2����20gϡ���������ʵ�����Ϊx����
Mg + H2SO4 == MgSO4 + H2��
98 2
x 0.12g
![]() =
=![]() �����x=5.88g
�����x=5.88g
ϡ���������ʵ����������ǣ� ![]() ��100%=29.4%
��100%=29.4%
��3�������������þ��������y.
Mg + H2SO4 == MgSO4 + H2��
24 2
y 0.4g
![]() =
=![]() �����y=4.8g
�����y=4.8g
��������������þ������Ϊ��10g-4.8g=5.2g������þ��þԪ�ص�����Ϊ��5.2g��![]() =1.04g�� ����������þԪ�ص���������Ϊ��
=1.04g�� ����������þԪ�ص���������Ϊ�� ![]() ��100%= 58.4%
��100%= 58.4%
��4����Һϡ��ǰ�����ʵ�������ȣ���60g������������Ϊ98%��Ũ���ᣬ��������ʵ����������������������ϡ���ᣬ���ˮ��������w��
60g��98%=��60g+w����29.4% �����w=140g

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�