��Ŀ����
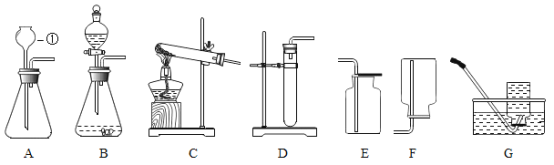
����Ŀ����ˮú���ǹ�ҵ�ϳɰ���ԭ����������Ҫ�ɷ���H2��CO��CO2��N2��ˮ������ij��ѧ��ȤС��Ϊ�����ˮú����ijЩ�ɷ֣���Ƶ�ʵ�鷽������ͼ��ʾ��
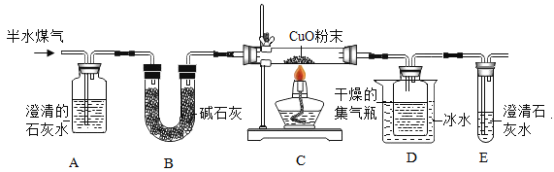
�������Ͽ�֪��ʯ����CaO��NaOH�Ļ����ش��������⣺
��1��Aƿ�в�����ɫ������֤����ˮú���к���_____����Ӧ�Ļ�ѧ����ʽ��____��
��2��Bװ�õ�������____��Cװ�õ�Ӳ�ʲ������з����û���Ӧ�Ļ�ѧ����ʽ��____��
��3��֤����ˮú���к���H2��������_____��
��4��֤����ˮú���к���CO��������____��
��5���ⶨ��ˮú����H2��CO������ʵ�鷽�����£�

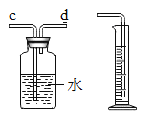
���������Ũ������������ag���������KOH��Һ��������bg�����ˮú����H2��CO��������Ϊ______���ú���ĸa��b�Ĵ���ʽ��ʾ����
���𰸡�CO2���������̼�� CO2��Ca(OH)2=CaCO3����H2O ���ն�����̼��ˮ���� H2��CuO![]() Cu��H2O C�к�ɫ��ĩ��죬D�м���ƿ�ڱ�����������Һ�� C�к�ɫ��ĩ��죬E�в�����ɫ���� 11a/63b
Cu��H2O C�к�ɫ��ĩ��죬D�м���ƿ�ڱ�����������Һ�� C�к�ɫ��ĩ��죬E�в�����ɫ���� 11a/63b
��������
��1��Aƿ�в�����ɫ������֤����ˮú���к��ж�����̼����Ӧ�Ƕ�����̼���������Ʒ�Ӧ����̼��ƺ�ˮ����ѧ����ʽ��CO2��Ca(OH)2=CaCO3����H2O��
��2����ʯ���ܹ����ն�����̼��ˮ��Bװ�õ����������ն�����̼��ˮ������Cװ�õ�Ӳ�ʲ������з������û���Ӧ��������ԭ����ͭ����ͭ��ˮ��һ����̼��ԭ����ͭ�����û���Ӧ���û���Ӧ�Ļ�ѧ����ʽ��. H2��CuO![]() Cu��H2O��
Cu��H2O��
��3��֤����ˮú���к���H2��������C�к�ɫ��ĩ��죬D�м���ƿ�ڱ�����������Һ�Ρ�
��4��֤����ˮú���к���CO��������C�к�ɫ��ĩ��죬E�в�����ɫ������
��5����������Ũ������������ag����������ԭ����ͭ����ˮag����������KOH��Һ��������bg����һ����̼��ԭ����ͭ���ɶ�����̼bg���μӷ�Ӧ����������Ϊ2ag/18=![]() ,�μӷ�Ӧ��һ����̼������Ϊ28bg/44=
,�μӷ�Ӧ��һ����̼������Ϊ28bg/44=![]() �����ˮú����H2��CO��������Ϊ
�����ˮú����H2��CO��������Ϊ![]() ��
��![]() =11a/63b��
=11a/63b��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�