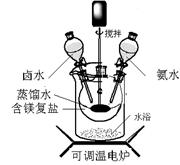
��Ŀ����
Cu��Zn�ĺϽ��Ϊ��ͭ���������ĵ����Ժ���ʴ�ԣ������������������������ij��ѧ��ȤС���ͬѧΪ�˲ⶨij��ͭ����ɣ�ȡ20g�û�ͭ��Ʒ���ձ��У������з�5�μ�����ͬ��������������ϡ���ᣬʹ֮��ַ�Ӧ��ÿ������ϡ�����������ʣ������������¼���±���
�Իش��������⣺
��1������������m��ֵΪ ��1�֣���
��2����ͭ��Ʒ��п����������Ϊ ��2�֣���
��3������ϡ��������������������Ƕ��٣�����д��������̣���3�֣�
| | ����ϡ�����������g�� | ��ַ�Ӧ��ʣ������������g�� |
| ��1�� | 20 | 17.4 |
| ��2�� | 20 | 14.8 |
| ��3�� | 20 | 12.2 |
| ��4�� | 20 | 12.0 |
| ��5�� | 20 | m |
��1������������m��ֵΪ ��1�֣���
��2����ͭ��Ʒ��п����������Ϊ ��2�֣���
��3������ϡ��������������������Ƕ��٣�����д��������̣���3�֣�
��1��12 ��1�֣� ��2��40% ��2�֣���3��19.6%
�����������1���ɱ������ݿ�֪��ÿ����20gϡ���ᣬ������Ӧ2.6gп������4�ε�ʱ����ֻ������0.2g��˵����ʱп�Ѿ�ȫ����Ӧ���ˣ��ʵ�5�μ������ᣬ������������ı䣬����m=12g��
��2����ͭ��Ʒ��п����������Ϊ
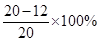 =40%��
=40%����3����20gϡ������ȫ��Ӧ��п������ = 20g-17.4g=2.6g
�⣺�裬20gϡ������H2SO4������Ϊx��
Zn + H2SO4 = ZnSO4 + H2��
65 98
2.6g x
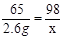
x=3.92g
����ϡ���������ʵ���������
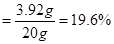
���������ݻ�ѧ����ʽ���㣬Ҫע�����IJ��裬�衢д���ҡ��С��⡢��
������������=
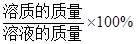 ��
��
��ϰ��ϵ�д�
 ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
�����Ŀ