��Ŀ����
(1)�����ֶ������Ͻ����к�̼���ϸߵ��� ��
(2)���dz��á�ͭǽ���ڡ�����������ļ�̣�������һ��������Ҳ�ܷ������ַ�Ӧ������˿�ܹ���������ȼ�գ��÷�Ӧ�Ļ�ѧ����ʽ�� ��
(3)��mg����ͭ��ĩ����μ���ijŨ�ȵ�ϡ���ᣬ�������ϡ����Ϊ100gʱ����ͭǡ����ȫ�ܽ⣬�õ���ɫ��ҺA������ҺA���ټ���һ���������ۣ���ַ�Ӧ�����ˣ��õ�����B����ҺC��������B�м���������ϡ���ᣬ������ð������ַ�Ӧ��ʣ��������ʵ�����Ϊ12.8g��
������B�Ļ�ѧʽ�� ����ҺC�����ʵ������� ��
��m�� ��
����ʽ����ϡ���������ʵ�����������
����ɫ��ҺA�����ʵ��������� ����ȷ��С�����һλ����
(2)���dz��á�ͭǽ���ڡ�����������ļ�̣�������һ��������Ҳ�ܷ������ַ�Ӧ������˿�ܹ���������ȼ�գ��÷�Ӧ�Ļ�ѧ����ʽ�� ��
(3)��mg����ͭ��ĩ����μ���ijŨ�ȵ�ϡ���ᣬ�������ϡ����Ϊ100gʱ����ͭǡ����ȫ�ܽ⣬�õ���ɫ��ҺA������ҺA���ټ���һ���������ۣ���ַ�Ӧ�����ˣ��õ�����B����ҺC��������B�м���������ϡ���ᣬ������ð������ַ�Ӧ��ʣ��������ʵ�����Ϊ12.8g��
������B�Ļ�ѧʽ�� ����ҺC�����ʵ������� ��
��m�� ��
����ʽ����ϡ���������ʵ�����������
����ɫ��ҺA�����ʵ��������� ����ȷ��С�����һλ����
��1������ ��2��3Fe+2O2 Fe3O4
Fe3O4
��3����Cu��Fe �������� ��m=16g
��19.6% ��27.6%
 Fe3O4
Fe3O4��3����Cu��Fe �������� ��m=16g
��19.6% ��27.6%
�����������1�������ֶ������Ͻ����������ĺ�̼��Ϊ2-4.3%���ֵĺ�̼��Ϊ0.03-2%���ʺ�̼���ϸߵ���������
��2����˿��������ȼ�գ������������������ʸ÷�Ӧ�Ļ�ѧ����ʽ��3Fe+2O2
 Fe3O4��
Fe3O4����3����������ͭ��ϡ���ᷴӦ����������ͭ��ˮ��������ͭǡ����ȫ��Ӧʱ���õ���ɫ��ҺAΪ����ͭ��Һ����������ͭ��Һ���ټ���һ���������ۣ����߳�ַ�Ӧ����ͭ�������������ʹ��˺õ�����B��һ������ͭ��������������B�м���������ϡ���ᣬ������ð����˵�����л�����ʣ�����������ҺCΪ���ɵ�����������Һ��
������ͭ�������Ӧ�����Գ�ַ�Ӧ��ʣ���12.8g����ȫ����ͭ������Ϊͭ��������������ͭ�е�ͭԪ�أ����У�m��
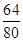 ��100%=12.8g�����m=16g��
��100%=12.8g�����m=16g���۸���������д��Ӧ�Ļ�ѧ����ʽ������������ͭ������������ȣ�������μӷ�Ӧ�Ĵ����������������������������������ʽ�����ϡ��������ʵ������������ɡ�
�⣺��ϡ���������ʵ�����Ϊx
CuO + H2SO4 = CuSO4 + H2O
80 98 160
16g x y
80:98=16g��x
��ã�x=19.6g
��ϡ���������ʵ���������=
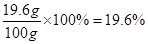
����������Ļ�ѧ��Ӧ����ʽ������������ͭ������ͭ�������ȣ���������ɵ�����ͭ������������������������������ʽ���������ͭ�����ʵ�������������
��80:160=16g��y
��ã�y=32g
��CuSO4��������������=
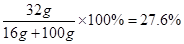
�����������Ĺؼ��ǣ����������غ㶨�ɣ�����μӷ�Ӧ������ͭ����������������֪����δ֪��Ӧ�������������㼴�ɣ�ע�����Ҫ�淶��

��ϰ��ϵ�д�
�����Ŀ
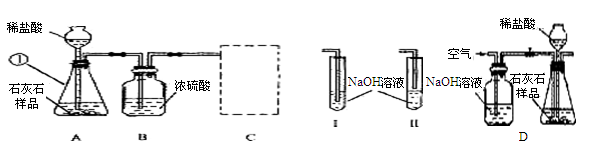
 C(���ʯ)+4NaCl������ɹ�����ѧ��������Ϊ�����ݱ�ƽ𡱡� ��Ҫ�Ƶ�1.2g���ʯ��ĩ,����Ҫ�����Ƶ�������
C(���ʯ)+4NaCl������ɹ�����ѧ��������Ϊ�����ݱ�ƽ𡱡� ��Ҫ�Ƶ�1.2g���ʯ��ĩ,����Ҫ�����Ƶ�������