��Ŀ����
����Ŀ����ľ���к�KC1��K2CO3�����ʣ�Ϊ�ⶨ���е�K2CO3�����������KCl��K2CO3�⣬����δ֪���ʲ�����ˮ��Ҳ�������ᷴӦ����������ͼʵ�飺��ش��������⣺

��1��ʵ�������Ҫ����100g������������Ϊ10%��ϡ���ᣬ�������������Ϊ25%��Ũ�������ơ���Ҫ����ˮ������Ϊ_____��
��2��д����Ӧ�Ļ�ѧ����ʽ_____��
��3���������⣬�г���������Ȼ��ص�������X���ı���ʽ_____��
��4���ò�ľ���к���K2CO3����������Ϊ_____��
��5�����յõ��IJ�������Һ�����ʵ���������Ϊ_____��
���𰸡��������1��60g
�������2��![]()
�������3��![]() ��
��![]()
�������4��6.9%
�������5��8%
��������
��1��ʵ�������Ҫ����100g������������Ϊ10%��ϡ���ᣬ�������������Ϊ25%��Ũ�������ơ���ҪŨ���������=![]() ����Ҫ����ˮ������Ϊ100g-40g=60g��
����Ҫ����ˮ������Ϊ100g-40g=60g��
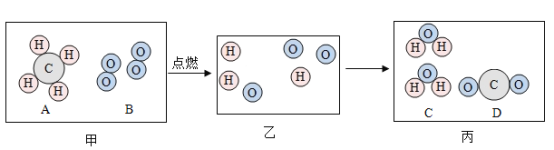
��2��̼�����ϡ���ᷴӦ�����Ȼ��ء�ˮ�Ͷ�����̼��Ӧ�Ļ�ѧ����ʽ![]() ��
��
��3���������Ȼ��ص�����Ϊx���μӷ�Ӧ̼��ص�����Ϊy������������̼���������Ϊz��
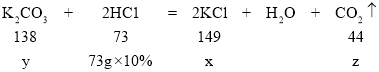
![]() x=14.9g
x=14.9g
![]() y=13.8g
y=13.8g
![]() x=4.4g
x=4.4g
�����Ȼ��ص�������X���ı���ʽ��![]() ��
��
��4���ò�ľ���к���K2CO3����������=![]() ��
��
��5�����յõ����Ȼ��ص���Һ�����ʵ���������=![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
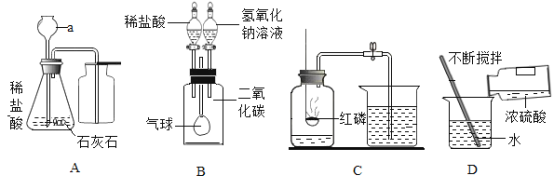
Сѧ��10����Ӧ����ϵ�д�����Ŀ���������װ�ûش����⡣
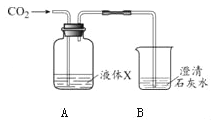
������a��������_________________��
��װ��B�еĻ�������______ ��ѡ��������رա���״̬��
��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ______�� ����װ��E�ռ�������̼������ʱȼ�ŵ�ľ��Ӧ����______��ѡ�m����n�����ˡ�
�ܻ�ѧ��Ȥ���Ž�����ѧ��ʵ���ֶ���װ��A̽������������Թ�������ֽ����ʵ�Ӱ�족�����±��������ʵ��һ�� ��ͬһ�¶���ͨ��ѹǿ�������õ�����������ͼ��ʾ��

ʵ��һ | ҩƷ |
��һ�� | 4%H2O2��Һ15mL |
�ڶ��� | 4%H2O2��Һ15mL��0.2gMnO2��ĩ |
������ | 4%H2O2��Һ15mL��0.2gFeCl3��ĩ |
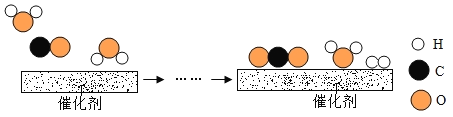
I .��������ʵ�飬ͬѧ�Ǵ�ʵ��һ�еó����ۣ� ������������ͬ������£�____ �����������ƣ��������Ĵ�Ч����á�
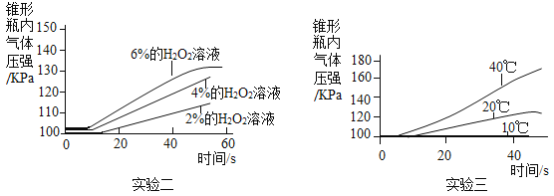
II .ѡ��0.2g��MnO2��ĩ��������ͬѧ���ֱַ�����ʵ�����ʵ�������õ�������ͼ�����ϣ�����ΪӰ��÷�Ӧ���ʵ�������س������⣬����_________��
��.ʵ��һ��ʵ�����4%�Ĺ���������Һ��0.2g MnO2��ĩ��Ϻ����������ѹǿ�����Բ�ͬ����ͼ��A���B�㣩������Ϊ���ܵ�ԭ����_________��
��С��ͬѧ�����ù���������ȡ0.1mol��������Ҫ������������ʵ���Ϊ����_____?�����ݻ�ѧ����ʽ���㣩