��Ŀ����
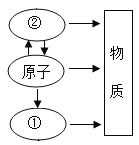
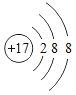
����Ŀ��ijʵ��С������ȡ����ͭ��֤������ͭ�ܼӿ�����صķֽ⣬����������ʵ�飺
��һ����ȡ����ͭ
�ٳ�ȡ2 g ��������ϸ�����ձ�����10 mL����ˮ�ܽ⣻
��������CuSO4��Һ����μ���NaOH��Һ��ֱ�����ٲ���������Ȼ�����û����ת�Ƶ���������������ȫ����Ϊ��ɫ��
�۽���������û������ˡ�ϴ�ӣ����ɺ���ϸ���á�
��1������ʵ�鲽������Ҫʹ�ò���������____��ѡ��ʵ����ţ�������١�������ĥ��������������������_____��
��2���������ϴ�ӳ����IJ�����_____��
������֤������ͭ�ܼӿ�����صķֽⲢ��������̵Ĵ�Ч�����бȽ�
����ͼװ�ý���ʵ�飬ʵ��ʱ��������25 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԣ�������ݼ��±���
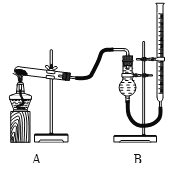
ʵ����� | KClO3���� | ������������ | �������� |
�� | 1.2 g | ���������� | |
�� | 1.2 g | CuO 0.5 g | |
�� | 1.2 g | MnO2 0.5 g |
��3������ʵ���еġ��������ݡ�ָ___��
��4����Ҫ֤��ʵ����и�������ռ���������O2���ɴ������ռ��������õ��ɼм�סA��B֮����齺�ܣ���ȥ������ϵ�����Ƥ����______��
��5��Ϊ̽��CuO��ʵ������Ƿ�������ã����貹����������ʵ�飺
a ____�������������̼�Ŀ�ģ���
b֤��CuO�Ļ�ѧ������û�иı䡣
���𰸡��٢ڢ� �в� �ز�������������м�����ˮ����û����������ʹ��ȫ���˳����ظ�2��3�� ����25 mL���������ʱ�� ȡһ�������ǵ�ľ�������������ڣ��۲�ľ���Ƿ�ȼ ȡʵ��ݲ���������������ˮ�У���ֽ��裬���ˣ�ϴ�Ӻ�ɫ����2��3�Σ���ɣ�������ɫ������������0.5 g�Ƚϣ���֤��CuO�������ڷ�Ӧǰ��û�иı�
��������
��1�������ܽ�����ʱ��Ҫ�ò������������ӿ����ʵ��ܽ⣬������Ҫʹ�ò�����������ת�Ƶ��������У����й���ʱʹ�ò���������������٢ڢۣ�
��ʵ�����г�ʹ���в�����״�����״������ϸ�������в���
��2��ϴ�ӳ����ķ������ز�������������м�����ˮ����û����������ʹ��ȫ���˳����ظ�2��3�Σ������ز�������������м�����ˮ����û����������ʹ��ȫ���˳����ظ�2��3�Ρ�
��3�������Ĵ�Ч��Խ�ã�����ȡ����������ķ�Ӧʱ��Խ�̣�����ͼ�еĴ��������Dz���25 mL���������ʱ�䣬��������25 mL���������ʱ�䡣
��4������������ȼ�ԣ��ܹ�ʹ�����ǵ�ľ����ȼ������֤��ʵ����и�������ռ���������O2�ķ����Ǵ������ռ��������õ��ɼм�סA��B֮����齺�ܣ���ȥ������ϵ�����Ƥ����ȡһ�������ǵ�ľ�������������ڣ��۲�ľ���Ƿ�ȼ������ȡһ�������ǵ�ľ�������������ڣ��۲�ľ���Ƿ�ȼ��
��5�������ڻ�ѧ��Ӧǰ���������ͻ�ѧ���ʱ��ֲ��䣬̽��CuO��ʵ������Ƿ�������ã�Ӧ���ڷ�Ӧ����֤����ͭ�������ͻ�ѧ�����Ƿ�ı䣬��֤����ͭ�������Ƿ�ı�ķ�����ȡʵ��ݲ���������������ˮ�У���ֽ��裬���ˣ�ϴ�Ӻ�ɫ����2��3�Σ���ɣ�������ɫ������������0.5 g�Ƚϣ���֤��CuO�������ڷ�Ӧǰ��û�иı䣬����ȡʵ��ݲ���������������ˮ�У���ֽ��裬���ˣ�ϴ�Ӻ�ɫ����2��3�Σ���ɣ�������ɫ������������0.5 g�Ƚϣ���֤��CuO�������ڷ�Ӧǰ��û�иı䡣

����Ŀ��ʵ������һƿ�ڿ�����¶�õ��������ƹ�����Ʒ��ij��ȤС���ͬѧ�Ը���Ʒ�ijɷּ�����������̽����
���������1������Ʒ�к�����Щ���ʣ�
��������룩ͨ��������������²��룺
�������Ʒ��ֻ��Na2CO3��
�������Ʒ�к���NaOH��Na2CO3��
�����__________________��
��NaOH����ԭ��Ļ�ѧ����ʽΪ__________________��
���������ϣ�
�ټ��Ե�Na2CO3��Һ���������Ե�CaCl2��Һ�������ֽⷴӦ��
��CO2�ڱ��͵�̼��������Һ�м������ܽ⡣
��ʵ��̽��1��
Ϊȷ������Ʒ�ijɷ֣�С�����������ʵ�鷽��������һ���������ʵ�鱨�档
ʵ����� | ʵ������ | ʵ����� |
(1)ȡ������Ʒ����ˮ���������� CaCl2��Һ | ______________ | ֤������������÷�Ӧ�Ļ�ѧ����ʽΪ__________ |
(2)��������Ӧ��Ļ��Һ���ˣ�ȡ��Һ����____________�� | ��Һ��� | ֤���������� |
��ʵ�鷴˼��
Ҫ��ȥNaOH�е�Na2CO3Ӧѡ��Ļ�ѧ�Լ���_____________��
���������2����β����ò��ֱ�����Ʒ��̼���Ƶ�����������
��ʵ��̽��2��
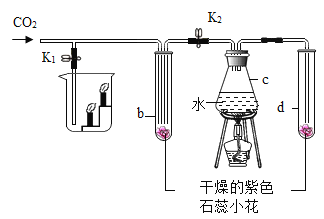
С��ͬѧ�����ͼ��ʾ��װ��(����̨��ȥ)��ʵ����27�棬101kPa �½��У��������£�
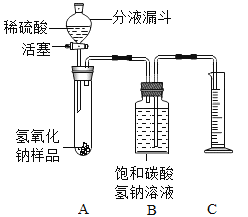
�ٰ�ͼ���Ӻ�װ�ã�
����������ƽȷ��ȡ����Ʒ2g������A���Թ��ڣ���B�м���ƿ�е��뱥�͵�̼��������Һ��ƿ������
�����Һ©���м���ϡ���ᣬ��������ϡ��������Թ������������رջ�������Ӧ��������Ͳ���ռ������͵�̼��������Һ244mL��
��ش��������⣺
��1����ʵ�鲽��ٺ͢�֮�䣬��ȱ��һʵ�鲽�裬��ʵ�鲽����_______________��
��2��B�м���ƿʢװ�ı��͵�̼��������Һ������ˮ���棬��������_______________��
��3���Թ���ԭ�еĿ�����ʵ�����Ƿ�������Ӱ�죿______________��������������û��������
��4����������������Ʒ��̼���Ƶ���������Ϊ________________������27�棬101kPaʱ������̼���ܶ�Ϊ1.8g/L������������ȷ��1%��
����Ŀ����ѧʵ��С���ͬѧ��ʵ�������������ƿ���ڷ����Ѿõ�NaOH��Һ�����Ƕ����ʵijɷֲ�����̽����������
[�������]��ƿNaOH��Һһ��������,����ʳ̶������?
[�������]����һ:NaOH��Һ���ֱ���
�����:NaOH��Һ��ȫ����
[ʵ��̽��]
��1������������Һ���ʵ�ԭ��Ϊ_____(�û�ѧ����ʽ��ʾ)��
��2��С���ͬѧ���������ʵ�����̽��,����ݱ���������д:
ʵ�鲽�� | ���� | ���� | |
����һ | ȡ����NaOH��Һ��Ʒ���Թ��У������еμӹ�����CaCl2��Һ | ������ɫ���� | ����__���� |
����� | ����,����Һ�еμӷ�̪��Һ | ��Һ���ɫ | |
��������һ��,�μӹ���CaCl2��Һ��Ŀ����____;������и��ݷ�̪��Һ�����һ����֤����Ʒ�к���___(����ĸ)��
A CaCl2
B Na2CO3
C NaOH
D Na2CO3��NaOH
[ʵ�鷴˼]
��1�����������Т�BaCl2��Һ����Ca( NO3)2��Һ����Ca(OH)2��Һ����Ba(OH)2��Һ,�����������һ��CaCl2��Һ����____(�����)��
��2���������������һ���Լ������̪��ҺҲ�ܵó�ͬ���Ľ��ۣ�������Ӧ�Ļ�ѧ����ʽΪ________��
��3��ͨ��̽��,ʵ�����е���������Ӧ�ܷⱣ�档