��Ŀ����
�����ڼ�����Ŀ�����������õĹ��˲�ʹ�õ��Ǿ��������ľ۱�ϩ[(C3H6)n]���粼��ij�о���ѧϰС��Ϊ��̽���۱�ϩ��������ȼ�յIJ�����۱�ϩ��һ�����������е�ȼ�������Ļ�������п����ж�����̼��һ����̼��ˮ���������û������ͨ�����µ�װ�ý���ʵ�飬����գ�
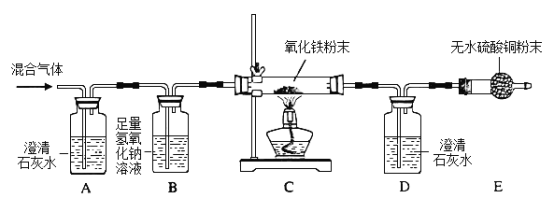
��1��ʵ���й۲쵽A��Dװ���г���ʯ��ˮ������ǣ�˵�������к���________________________��
��2��Bװ�õ�������________________________��
��3��д��Cװ���з�����Ӧ�Ļ�ѧ����ʽ________________________��
��4����ͬѧ�����ѧϰС����Ƶ�װ��E�������������齫Eװ�û���____________��
��5������ʵ���������ѧ֪ʶ�����������ó���ʵ���о۱�ϩ��ȼ�ղ���Ϊ____________����д��ѧʽ����
Ϊ�ⶨijCuSO4��Һ�����ʵ��ʷ�����ȡ150g CuSO4��Һ��ƽ����Ϊ���ݣ�ÿ����Ʒ������ͼ��ʾ����ʵ�飬ʵ�����ݼ��±�����ѧ����ʽ��CuSO4 + 2NaOH = Cu(OH)2�� + Na2SO4
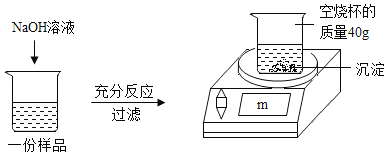
ʵ��1 | ʵ��2 | ʵ��3 | |
��Ʒ����/g | 50 | 50 | 50 |
NaOH��Һ����/g | 20 | 40 | 60 |
���ӳӶ���m/g | 42.45 | 44.9 | 44.9 |
����㣺
��1��50g CuSO4��Һ��ȫ��Ӧʱ�����ó���������Ϊ�� ��g��
��2��CuSO4��Һ�����ʵ�����������



