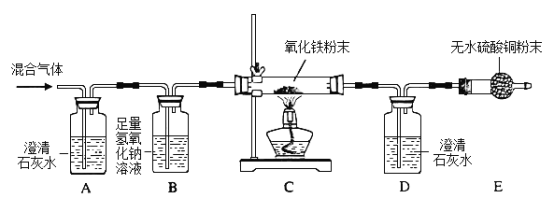
��Ŀ����
Ϊ�ⶨijCuSO4��Һ�����ʵ��ʷ�����ȡ150g CuSO4��Һ��ƽ����Ϊ���ݣ�ÿ����Ʒ������ͼ��ʾ����ʵ�飬ʵ�����ݼ��±�����ѧ����ʽ��CuSO4 + 2NaOH = Cu(OH)2�� + Na2SO4
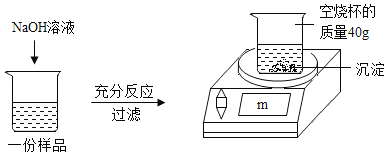
ʵ��1 | ʵ��2 | ʵ��3 | |
��Ʒ����/g | 50 | 50 | 50 |
NaOH��Һ����/g | 20 | 40 | 60 |
���ӳӶ���m/g | 42.45 | 44.9 | 44.9 |
����㣺
��1��50g CuSO4��Һ��ȫ��Ӧʱ�����ó���������Ϊ�� ��g��
��2��CuSO4��Һ�����ʵ�����������
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
���ж�֪ʶ�������д����һ����
A.��ѧ�뽡�� ���ˮ�������ڲ���ά���� ��ҵ�Σ��������ƣ������߲� | B.��ѧ��ȼ�� ��ʯȼ��ȼ����ɿ�����Ⱦ úȼ���ŷų�������������������Ⱦ�� |
C.��ѧ��Ӧ�� ��ʳ�׳�ȥˮ���е�ˮ�� �û���̿��ȥ�����е���ζ | D.��ѧ�밲ȫ ��ȼ��ȼ������ǰ���鴿 �����δ�����IJ˽��������ƻ����顱 |
A.A B.B C.C D.D