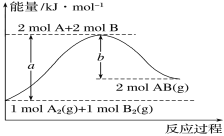
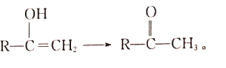
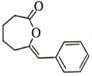
ΧβΡΩΡΎ»ί
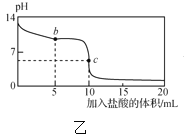
ΓΨΧβΡΩΓΩ “Έ¬œ¬Θ§ΫΪ1.00molΓΛL-1―ΈΥαΒΈ»κ20.00mL1.00molΓΛL-1ΒΡΑ±Υ°÷–Θ§»ή“ΚpHΚΆΈ¬Ε»ΥφΦ”»κ―ΈΥαΧεΜΐΒΡ±δΜ·«ζœΏ»γΆΦΥυ ΨΓΘ

œ¬Ν–”–ΙΊΥΒΖ®÷–≤Μ’ΐ»ΖΒΡ «Θ® Θ©
A. aΒψ»ή“Κ÷–άκΉ”≈®Ε»¥σ–ΓΙΊœΒΘΚcΘ®NH4+Θ©ΘΨcΘ®Cl-Θ©ΘΨcΘ®OH-Θ©ΘΨcΘ®H+Θ©
B. bΒψ»ή“Κ÷–άκΉ”≈®Ε»¥σ–ΓΙΊœΒΘΚcΘ®NH4+Θ©=cΘ®Cl-Θ©ΘΨcΘ®H+Θ©=cΘ®OH-Θ©
C. cΒψ»ή“Κ÷–άκΉ”≈®Ε»¥σ–ΓΙΊœΒΘΚcΘ®NH4+Θ©+cΘ®H+Θ©=cΘ®Cl-Θ©+cΘ®OH-Θ©
D. dΒψ ±»ή“ΚΈ¬Ε»¥οΒΫΉνΗΏΘ§÷°ΚσΈ¬Ε»¬‘”–œ¬ΫΒΘ§‘≠“ρ «NH3ΓΛH2OΒγάκ
ΓΨ¥πΑΗΓΩD
ΓΨΫβΈωΓΩ
A.aΒψ»ή“Κ÷–ΈΣΒ»Έο÷ ΒΡΝΩ≈®Ε»ΒΡ![]() ΚΆ
ΚΆ![]() Θ§“ρ»ή“Κœ‘Φν–‘Θ§Ι Α±Υ°ΒΡΒγάκ≥ΧΕ»¥σ”ΎNH4ClΒΡΥ°Ϋβ≥ΧΕ»Θ§Υυ“‘”–
Θ§“ρ»ή“Κœ‘Φν–‘Θ§Ι Α±Υ°ΒΡΒγάκ≥ΧΕ»¥σ”ΎNH4ClΒΡΥ°Ϋβ≥ΧΕ»Θ§Υυ“‘”–![]() Θ§Aœν’ΐ»ΖΘΜ
Θ§Aœν’ΐ»ΖΘΜ
B.bΒψ»ή“Κœ‘÷––‘Θ§”–![]() Θ§ΗυΨίΒγΚ… ΊΚψ”–
Θ§ΗυΨίΒγΚ… ΊΚψ”–![]() Θ§«“”–
Θ§«“”–![]() Θ§Bœν’ΐ»ΖΘΜ
Θ§Bœν’ΐ»ΖΘΜ
C.cΒψ»ή“Κ÷–»ή÷ «NH4ClΚΆHClΘ§ΗυΨίΒγΚ… ΊΚψ”–![]() Θ§ Cœν’ΐ»ΖΘΜ
Θ§ Cœν’ΐ»ΖΘΜ
D.dΒψ ±ΥαΦν«ΓΚΟ÷–ΚΆΘ§Ζ≈≥ω»»ΝΩΉνΕύΘ§»ή“ΚΈ¬Ε»¥οΒΫΉνΗΏΘ§÷–ΚΆΖ¥”ΠΫα χΚσΘ§Φ”»κΒΡ―ΈΥαΈ¬Ε»ΒΆΘ§ΥφΉ≈―ΈΥαΒΡΦ”»κΜλΚœ»ή“ΚΈ¬Ε»÷πΫΞΫΒΒΆΘ§ΗζNH3ΓΛH2OΒγάκΈόΙΊΘ§Dœν¥μΈσΘΜ¥πΑΗ―ΓDΓΘ

 ΫΧ―ßΝΖ–¬Ά§≤ΫΝΖœΑœΒΝ–¥πΑΗ
ΫΧ―ßΝΖ–¬Ά§≤ΫΝΖœΑœΒΝ–¥πΑΗ ΩΈ«ΑΩΈΚσΆ§≤ΫΝΖœΑœΒΝ–¥πΑΗ
ΩΈ«ΑΩΈΚσΆ§≤ΫΝΖœΑœΒΝ–¥πΑΗ ΩΈΧΟ–ΓΉς“ΒœΒΝ–¥πΑΗ
ΩΈΧΟ–ΓΉς“ΒœΒΝ–¥πΑΗ ΜΤΗ‘–ΓΉ¥‘ΣΩΎΥψΥΌΥψΝΖœΑ≤αœΒΝ–¥πΑΗ
ΜΤΗ‘–ΓΉ¥‘ΣΩΎΥψΥΌΥψΝΖœΑ≤αœΒΝ–¥πΑΗ ≥…ΙΠ―ΒΝΖΦΤΜ°œΒΝ–¥πΑΗ
≥…ΙΠ―ΒΝΖΦΤΜ°œΒΝ–¥πΑΗ