��Ŀ����
7�� ���ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000mol•L-1��HCl����Һ�����к͵ζ����ü�����ָʾ������
���ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000mol•L-1��HCl����Һ�����к͵ζ����ü�����ָʾ��������ش��������⣺
��1���ζ�ʱ��ʢװ����NaOH��Һ����������Ϊ��ƿ��
��2��ʢװ���������������Ϊ��ʽ�ζ��ܣ�
��3���ζ����յ����ɫ�仯Ϊ��Һ�ɻ�ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��
��4������ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ0.50mL���ζ���Һ����ͼ�����ʱ���ı���Һ�����Ϊ26.90mL��
��5����ѧ����������ƽ��ʵ�飬���ݼ�¼���£�
ѡȡ�����������ݣ����������NaOH��Һ�����ʵ���Ũ��Ϊ0.1052mol/L ��������λ��Ч���֣���
| ʵ����� | ����NaOH��Һ�����/mL | 0.1000mol•L-1HCl��Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 0.00 | 26.29 |
| 2 | 25.00 | 1.00 | 31.00 |
| 3 | 25.00 | 1.00 | 27.31 |
A����ƿ������ˮϴ�������ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ�����
���� ��1���ζ�������Һ�ڵζ����У�����Һʢ����ƿ�У�
��2��������Һ�������ʽ�ζ����У�
��3�����ݵζ��յ�ʱ��Һ��ɫ�ɻ�ɫͻ��Ϊ��ɫ���ұ��ְ���Ӳ���ɫ��
��4�����ݵζ��ܵĽṹ�뾫ȷ��Ϊ0.01mL��
��5���ȸ������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ�����Ÿ��������NaOH��Ӧ���C��NaOH����
��6������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��жϣ�
��� �⣺��1������ʽ�ζ���ȡ�������NaOH��Һ����ƿ�У�
�ʴ�Ϊ����ƿ��
��2��ʢװ���������������Ϊ��ʽ�ζ��ܣ�
�ʴ�Ϊ����ʽ�ζ��ܣ�
��3������Һ���������ƣ���ƿ��ʢ�е�����������Һ�е�����ȣ���Һ����ɫ�ǻ�ɫ��������Һ��pH��С�����ε���Һ��pHС��4.4ʱ����Һ��ɫ�ɻ�ɫ��ɳ�ɫ���Ұ���Ӳ���ɫ���ε�������
�ʴ�Ϊ����Һ�ɻ�ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��
��4����ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ0.50mL���ζ���Һ����ͼΪ27.40ml���ζ����е�Һ�����Ϊ27.40ml-0.50mL=26.90mL��
�ʴ�Ϊ��26.90mL��
��5���������ݵ���Ч�ԣ���ȥ��2�����ݣ���1��3��ƽ������V�����ᣩ=$\frac{26.29mL+26.31mL}{2}$=26.30mL��
HCl+NaOH�TNaCl+H2O
0.0263L��0.1000mol•L-1 0.025L��C��NaOH��
��C��NaOH��=$\frac{0.0263L��0.1000mol•{L}^{-1}}{0.025L}$=0.1052mol/L��
�ʴ�Ϊ��0.1052mol/L��
��6��A����ƿ������ˮϴ�������ô���Һ��ϴ����ʹ��ƿ�����ʵ����ʵ����������V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$���������c�����⣩ƫ�ߣ���A��ȷ��
B����ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ����V��������Ӱ�죬����c�����⣩=$\frac{c��������V������}{V�����⣩}$���������c�����⣩���䣬��B����
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ�������V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$���������c�����⣩ƫ�ߣ���C��ȷ��
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ����������V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$���������c�����⣩ƫ�ͣ���D����
��ѡAC��
���� ���⿼��������к͵ζ�ʵ��IJ������衢�ζ��ܵĽṹ���յ��ж��Լ�����Ӧ�ã��յ��жϣ���Ŀ�Ѷ��еȣ�

 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�| A�� | a��b | B�� | a��b | C�� | a=b | D�� | ��ȷ�� |
| A�� | 26�� | B�� | 27�� | C�� | 28�� | D�� | 29�� |
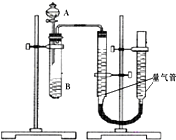 �ס�������С����������KMnO4��H2C2O4��Һ��Ӧ�����ʵ��̽��Ӱ�췴Ӧ���ʵ����أ�2MnO4-+5H2C2O4+6H+=2Mn2++10CO2+8H2O��
�ס�������С����������KMnO4��H2C2O4��Һ��Ӧ�����ʵ��̽��Ӱ�췴Ӧ���ʵ����أ�2MnO4-+5H2C2O4+6H+=2Mn2++10CO2+8H2O�����飺������ͼװ�ã�ͨ���ⶨ��λʱ��������CO2��������Ĵ�С���Ƚϻ�ѧ��Ӧ���ʵĴ�С����ʵ��������KMnO4��Һ���Ѽ���H2SO4��
| ��� | A��Һ | B��Һ |
| �� | 2ml 0.2mol/LH2C2O4��Һ | 4ml 0.01mol/LKMnO4��Һ |
| �� | 2ml 0.1mol/LH2C2O4��Һ | 4ml 0.01mol/LKMnO4��Һ |
| �� | 2ml 0.2mol/LH2C2O4��Һ | 4ml 0.01mol/LKMnO4��Һ������MnSO4 |
��2����Һ©����A��ҺӦ��һ���Լ��루�һ���ԡ�����εμӡ���
��3��ʵ���������ǰΪ��ʹ���������ܵ�ѹǿ��ȣ��������ѹǿ�Ӱ��ⶨ�������Ҫ���еIJ������ƶ������ܣ�ʹ���������ܵ�Һ����ƽ�����飺ͨ���ⶨKMnO4��Һ��ɫ����ʱ��Ķ������Ƚϻ�ѧ��Ӧ����Ϊ��̽��KMnO4��H2C2O4Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죬ijͬѧ���������������ʵ��
| ʵ���� | 1 | 2 | 3 | 4 |
| ˮ/ml | 10 | 5 | 0 | X |
| 0.5mol/L H2C2O4/ml | 5 | 10 | 10 | 5 |
| 0.2mol/L KMnO4/ml | 5 | 5 | 10 | 10 |
| ʱ��/s | 40 | 20 | 10 | --- |
A��5 B��10 C��15 D��20
4��ʵ����ʼ��û�й۲쵽��Һ��ɫ������Ϊ���ܵ�ԭ����KMnO4��Һ������
 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ���̪��ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ���̪��ָʾ��������д���пհף���1���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�ı仯���жϵ���ζ��յ�������ǣ���Һ�ɺ�ɫ��Ϊ��ɫ��������ڲ���ɫ��
��2�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���D
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ������ʼ����Ϊ0.00mL���յ����Ϊ26.10mL������������Һ�����Ϊ26.10mL��
��4��ijѧ������3��ʵ��ֱ��¼�й����������
| �ζ� ���� | ����NaOH��Һ�����/mL | 0.100 0mol/L��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
| A�� | O2��O3 | B�� | 2H2��3H2 | ||
| C�� | H2O��H2O2 | D�� | C2H5COOH��CH3COOCH3 |
 һ�������������Ժ�CO2������Ӧ��Fe��s��+CO2��g��?FeO��s��+CO��g����H��0��1 100��ʱ����ij�ܱ������м����������۲�����һ������CO2���壬��Ӧ������CO2��CO��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
һ�������������Ժ�CO2������Ӧ��Fe��s��+CO2��g��?FeO��s��+CO��g����H��0��1 100��ʱ����ij�ܱ������м����������۲�����һ������CO2���壬��Ӧ������CO2��CO��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ����1���÷�Ӧ��ƽ�ⳣ������ʽK=$\frac{c��CO��}{c��C{O}_{2}��}$��
��2�����д�ʩ����ʹƽ�ⳣ��K�������a������ţ�
A�������¶� B������ѹǿ C������һ����CO D�������¶�
��3��8min�ڣ�CO��ƽ����Ӧ����v��CO��=0.0625mol•L-1•min-1��
��4��1 100��ʱ��2L���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������£�
| ���� | �� | �� |
| ��Ӧ��Ͷ���� | 3mol Fe��2mol CO2 | 4mol FeO��3mol CO |
| CO��Ũ�ȣ�mol•L-1�� | c1 | c2 |
| CO2��������� | ��1 | ��2 |
| ��ϵѹǿ��Pa�� | p1 | p2 |
| ��̬��Ӧ���ת���� | ��1 | ��2 |
A��2c1=3c2 B����1=��2 C��p1��p2 D����1=��2
����c1=0.67mol•L-1����1=33.3%����2=55.5%��

 �����к��еĻ�ѧ���й��ۼ������Ӽ����������� ����
�����к��еĻ�ѧ���й��ۼ������Ӽ����������� ���� ��
��