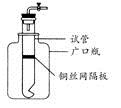
��Ŀ����
һλѧ����������������Ӧ��IJ������̽����
(1)�������
����1������ΪFeO��
����2�� ��
(2)��������
��ѧ��ͨ���������ϵ�֪�������������������У��������������ȶ�������������ȶ��������¼��ױ�����������������(��ɫ�ɺ�ɫ��ɺ�ɫ)��
ͨ���������Ͽ��Եó��ij�������Ϊ ��
(3)����ʵ��
��ѧ�����������װ�ý���������������Ӧ��ʵ�顣�����������ʵ�鲽�貹��������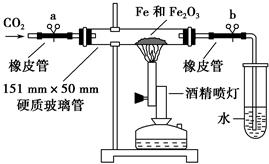
�ٰ���ͼװ�����Ӻ�����(�ݲ�װ��ҩƷ)�� ��
�ڳ�ȡ1 g��ԭ�����ۺ�5 g��������ĩ����Ͼ��Ⱥ�ƽ̯�ڲ������в���
���ɿ��������ɼУ� �����ɼ��ϵ��ɼ�a������ʼ����ҩƷ��
�ܴ�Լ4�������ң���ɫ��ĩȫ����ڣ��ټ��ϵ��ɼ�b��Ȼ��ֹͣ���ȣ��ȵ���������ȴ�����£�������ɫ��ĩ��
(4)���ṩ����ҩƷ����֤ʵ��õ��ĺ�ɫ��ĩ�ijɷ֡�������ϡ���ᡢKSCN��Һ������KMnO4��Һ���Թܡ���ͷ�ιܡ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| | |
(5)ʵ����ۣ�������������Ӧ�Ļ�ѧ����ʽΪ ��
(1)����ΪFe3O4
(2)Fe��Fe2O3��Ӧ�IJ���ΪFe3O4(������������)
(3)�ټ��ϵ��ɼ�a���ɿ����ɼ�b���Ȳ����ܣ������ܿ�������ð����ֹͣ����һ��ʱ��������γ�һ��ˮ������װ�ò�©�� ��ͨ�봿������Ķ�����̼���壬����������Ŀ�����������
(4)
(5)Fe��4Fe2O3ʵ�鲽�� Ԥ������ͽ��� ����1���ô���������ɫ��ĩ ��ɫ��ĩ�ڿ����в���ɫ�����ܱ�����ȫ��������˵����ɫ��ĩ��û��FeO ����2��ȡ������ɫ��ĩ�������Թ��У�����ϡ���ᣬ��ʹ��ĩ�ܽ⣬Ȼ����뼸��KSCN��Һ ������ð������Һ���ɫ��˵�����۹�����������ΪFe3O4  3Fe3O4
3Fe3O4
����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���15�֣�Fe2O3�׳��������죬������������ɫ������ҵ��ú��ʯ����Ҫ�ɷ֣�SiO2 49.5%��Fe2O3 20.6%��Al2O318.9%���Լ�MgO��FeO���������ʣ��Ʊ����о����������Ӧ�á�
��һ���Ʊ���������
1��Ԥ��������ú��ʯ���飬��350���±���2Сʱ��
2�������ܽ⣺��Ԥ�������ú��ʯ������������Ϊ15%������������Һ�У����ˡ�����Һ�м����H2O2��
3������pH����������Һ�м�1mol/LNaOH��Һ������Һ��pH���ٹ��ˣ��õ�������
4����Ʒ����������������ˮϴ����ɡ����ա���ĥ����ɸ�ò�Ʒ��
��֪����������������������ʽ����ʱ��Һ��pH���±�
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 |
| ��ʼ���� | 1.3 | 3.3 | 7.5 | 10.3 |
| ��ȫ���� | 2.8 | 5.2 | 9.7 | 12.2 |
��1��Ԥ����ʱ����ú��ʯ����2Сʱ��Ŀ���� ��
��2���������������H2SO4�������� ��
��ʵ�����н��иò���ʱ���õ����������� �� ��
��3����NaOH��Һ����pH����ѷ�Χ�� �����ӵڶ��ι��˵���Һ�л�ȡ�ϴ���������þ���壬Ӧ����IJ����� ��ϴ�Ӻ��T�á�
��4����Ʒ����ʱ��������ˮϴ�IJ����� ��
���������������ۣ�


��5����Ʒ����ʱ�������¶ȶԲ�Ʒ�Ĵ����кܴ�Ӱ�졣��֪�¶ȶԲ��﴿�ȵ�Ӱ����ͼ1��ʾ��������ʱ�¶���ÿ����� �档
��6���������Ƿ�ӳ��������������ʵ���Ҫָ�ꡣ��������˵���������������ϴ������������л��������ٳ���Ӱ����������ͬŨ�ȵ����ּ���Һ�Բ���������Ӱ����ͼ2��ʾ���������������ڵ�����ҺpHʱ��ѡ��NaOH��Һ����ѡ�ð�ˮ��ԭ���� ��
�ҹ�����ר�Һ�°����ڴ��£��Ľ��������ˡ������Ƽ����Ϊ�����Ƽҵ������ͻ�����ס�������������⣺
��1���������Ƽ���Ƶõġ���� (�ѧʽ)��
��2������������Ƽ��������Ҫ�Ĺ�ҵ�Ƽ�����б����У�����ȷ���� ��
| | | ��� | �����Ƽ |
| A | ԭ�� | ʳ�Ρ���������ʯ�� | ʳ�Ρ�������������̼ |
| B | ���ܵĸ����� | �Ȼ��� | �Ȼ�� |
| C | ѭ������ | ������������̼ | �Ȼ��� |
| D | ���� | ԭ���ã��豸���ӣ��ܺĸ� | ԭ�������ʸߣ��������� |
ijʵ��С�飬��������װ��ģ�⡰�����Ƽ����

��3��ȡ������������װ�ã�˳��Ϊ��(a)��( )��( )��( )��(b)��( )��
���������Ժ�װ��ҩƷ��Ӧ������ װ�ã���������ĸ���ȷ�����Ӧ��ֱ�����������岻������C���ܽ�ʱ����ͨ����һװ���в��������塣
��4��C�������θ���ܶ�����ֱ���ܣ��������� ��D��Ӧѡ�õ�Һ��Ϊ ��
��5��C�й��ƿ�ڲ���������ܻ�ѧ����ʽΪ ��
��6����Ʒ�����к���̼�����ơ�����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ����������ɱ�ʾΪ�� (ע����ı���ʽ�����õ��йط��ŵĺ���)��
�ú���Al2O3��SiO2������FeO��xFe2O3�������Ʊ�Al2(SO4)3��18H2O��������������(���ֲ�����������)��
��.�������м������ϡH2SO4�����ˣ�
��.����Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
��.���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ��
��.����MnSO4���Ϻ�ɫ��ʧ�����ˣ�
��.Ũ�����ᾧ�����룬�õ���Ʒ��
��1��H2SO4�ܽ�Al2O3�����ӷ���ʽ��____________________________________��
��2����MnO4-����Fe2�������ӷ���ʽ����������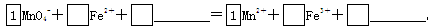
��3����֪��
�����������������pH
| | Al(OH)3 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
���ݱ������ݽ��Ͳ�����Ŀ�ģ�________��
��4����֪��һ�������£�MnO4-����Mn2����Ӧ����MnO2��
�� �� �� �ij����м���ŨHCl�����ȣ���˵�������д���MnO2��������__________��
�ڢ� �м���MnSO4��Ŀ����_____________________________________________��
����������FeC2O4�������������Լ�����Ӱ���Լ����͵�ز�����������﮵����������������ڸ�������ʱ�����ܹ��ֽ�,��ȤС��Բ��������ķֽ���������ʵ���̽��������֪��CO�����Ȼ���[PdC12]��Һ��Ӧ���ɺ�ɫ���ٷۡ���
��1�������������ֽ�������������ͨ������ʯ��ˮ���Ȼ�����Һ���۲쵽����ʯ��ˮ����ǣ��Ȼ�����Һ���к�ɫ�������ɡ�˵������������� �����ѧʽ��
��2��̽�����������ֽ�õ��ĺ�ɫ�����������Ԫ�صĴ�����ʽ��
���������⡿
���������ֽ��õ��ĺ�ɫ������ʲô��
��������衿
����1�� ������2��FeO������3��FeO��Fe�Ļ���
��ʵ�鷽����
��ѡ�Լ������ᡢ��ˮ��CuSO4��Һ��KSCN��Һ������ˮ��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ����1�����Թ��м��������������ټ������� ������� | ����Һ��ɫ���Ըı䣬 ���к�ɫ�������ɡ� | ��Fe���ڡ� |
| ����2��������1�еõ�����Һ���ˣ���������ˮ������ϴ����ϴ��Һ����ɫ�� | | |
| ����3��ȡ����2�õ��������������Թ��У��μӹ������ᣬ���ú�ȡ�ϲ���Һ�� �� | �� | ��FeO���ڡ� |
����˼������
����ȤС�����۷�����Ϊ����������ֱ�ӷֽ����ù������Ӧ����FeO�������չ�������л�����Fe����Ϊ ��д��ѧ����ʽ����
��3������ʵ��̽���ͷ�˼��д�����������ڸ�������ʱ����ֱ�ӷֽ�Ļ�ѧ����ʽ ��