��Ŀ����
16�� 25��ʱ����100mL��0.1mol•L-1��NH4HSO4��Һ�еμ�0.1mol•L-1��NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ����μӹ����������������������˵��������ǣ�������
25��ʱ����100mL��0.1mol•L-1��NH4HSO4��Һ�еμ�0.1mol•L-1��NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ����μӹ����������������������˵��������ǣ�������| A�� | δ�μ�NaOH��Һʱ��Һ��pHС����ͬ������0.1mol•L-1��NaHSO4��Һ��pH | |
| B�� | pHΪ7ʱ�����Һ��ˮ�ĵ���̶���� | |
| C�� | ��V��NaOH��=30mLʱ��c��NH3•H2O��+c��Na+����2c��SO42-�� | |
| D�� | �μ�NaOH��Һ�������30mL��40mL�Ĺ����У�$\frac{c��N{{H}_{4}}^{+}��}{c��{H}^{+}��}$��ֵ������ |
���� A��笠�����ˮ�����������ӣ�
B��NH4HSO4��NaOHǡ�÷�Ӧ���ɣ�NH4��2SO4��Na2SO4ʱ����Һ��ˮ�ĵ���̶����
C����V��NaOH��=30mLʱ����Һ������ΪNH4HSO4����NH4��2SO4��Na2SO4��
D���μ�NaOH��Һ�������30mL��40mL�Ĺ����У�NaOH���������ӣ�������Ũ�ȼ�С��
��� �⣺A��0.1mol•L-1��NH4HSO4��Һ�У�笠�����ˮ�����������ӣ�����NH4HSO4��Һ��������Ũ�ȴ�����ͬ������0.1mol•L-1��NaHSO4��Һ����0.1mol•L-1��NH4HSO4��Һ��pHС����ͬ������0.1mol•L-1��NaHSO4��Һ��pH����A��ȷ��
B��NH4HSO4��NaOHǡ�÷�Ӧ���ɣ�NH4��2SO4��Na2SO4ʱ������笠����ӷ���ˮ�⣬������Һ��ˮ�ĵ���̶����ʱ��Һ�����ԣ���B����
C����V��NaOH��=30mLʱ����Һ������ΪNH4HSO4����NH4��2SO4��Na2SO4����ʱ��Һ�����ԣ���Һ��ˮ�����ɵ�NH3•H2O��Ũ�Ⱥ�С������c��NH3•H2O��+c��Na+����2c��SO42-������C��ȷ��
D���μ�NaOH��Һ�������30mL��40mL�Ĺ����У�NaOH���������ӣ�������Ũ�ȼ�С����c��NH4+���������䣬����$\frac{c��N{{H}_{4}}^{+}��}{c��{H}^{+}��}$��ֵ������D��ȷ��
��ѡB��
���� ���⿼��������ϵĶ����жϼ�����Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ��Һ���������ҺpH�Ĺ�ϵΪ���ؼ���ע��ͼ���и�������������Һ�е����ʣ�����������ѧ���ķ������������Ӧ��������

 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д���HCN?H++CN-��H1��0 K1
��CN-+H2O?HCN+OH-��H2��K2
�����£�K1=6.2��10-10������������������ʵ���Ũ�ȵ�HCN��NaCN��Һ��ϣ�����������ȷ���ǣ�������
| A�� | K2��1.6��10-3 | B�� | 2c��Na+��=c��HCN��+c��CN-�� | ||
| C�� | �����Һ��pH��7 | D�� | �Ի����Һ���£�K1����K2��С |

| A�� | ������Ǧ���̰����ֽⷴӦ��������ԭ��Ӧ | |
| B�� | ұ��ʱп��Ϊ�����ڶ�������Ϊ���� | |
| C�� | ����⡱��Ŀ����Ϊ�˷�ֹ�õ���п������ | |
| D�� | ��п����п������һ����̼�ڿ�����ȼ�� |
| A�� |  | B�� |  | C�� |  | D�� |  |



 ��
�� +CH2=CHCH2OH$��_{��}^{Ũ����}$
+CH2=CHCH2OH$��_{��}^{Ũ����}$ +H2O��
+H2O�� ��
�� ��
��
 ��
�� ����дһ�֣��������������칹��
����дһ�֣��������������칹�� �����ᣨHClO������������Һ�У��к�ǿ�������Ժ�Ư�����ã�ij��ȤС������Cl2O�볱ʪNa2CO3��Ӧ�Ƶ�Cl2O������ˮ����Cl2O�Ʊ���������Һ�����ⶨ��Ũ�ȣ�
�����ᣨHClO������������Һ�У��к�ǿ�������Ժ�Ư�����ã�ij��ȤС������Cl2O�볱ʪNa2CO3��Ӧ�Ƶ�Cl2O������ˮ����Cl2O�Ʊ���������Һ�����ⶨ��Ũ�ȣ�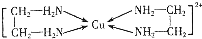 �������ڽ���Cr��Fe��Cu�ڿ�ѧ�о���ҵ�����ж�����Ҫ����;����ش��������⣺
�������ڽ���Cr��Fe��Cu�ڿ�ѧ�о���ҵ�����ж�����Ҫ����;����ش��������⣺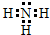 ��
��