��Ŀ����
12���Ի�����Ϊԭ����������Ĺ�������ͼ��ͼ��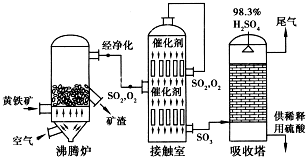
��1�������ջ�����Ļ�ѧ����ʽ����������4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2��
��2���Ӵ����з�����Ӧ�Ļ�ѧ����ʽ��2SO2+O2$?_{���¸�ѹ}^{����}$2SO3��
��3�����ݹ�������ͼ�ж�����˵����ȷ����a��b��d��ѡ�������ĸ����
a��Ϊʹ��������ȼ�գ��轫�����
b���������������SO2��ת����
c��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת����
d������¯�ų��Ŀ����ɹ�����
��4��ÿ160g SO3������H2O���Ϸų�260.6kJ���������÷�Ӧ���Ȼ�ѧ����ʽΪSO3��g��+H2O��l���TH2SO4��l������H=-130.3kJ/mol��
��5���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�����õ��ϸ�Ũ�ȵ�SO2����Σ�
��SO2�ȿ���Ϊ���������ԭ��ѭ�����ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2����Br2�����ӷ���ʽ��SO2+Br2+2H2O�T4H++2Br-+SO42-��
��Ϊ�ⶨ������е�Ԫ�ص���������������ͬ��������ηֱ���뵽50.00mL��ͬŨ�ȵ�NaOH��Һ�У���ˮԡ����������ȫ���ݳ������¶�����β��ֽ⣩�������徭�������Ũ����������ȫ���ⶨŨ�������ӵ�������
���ֲⶨ�����
�������Ϊ10.00g��20.00gʱ��Ũ�������ӵ�������ͬ���������Ϊ30.00gʱ��Ũ�������ӵ�����Ϊ0.68g���������Ϊ40.00gʱ��Ũ������������䣮����㣺
������е�Ԫ�ص�����������14.56%�����������Ϊ15.00g��Ũ�������ӵ�����Ϊ2.31 g��������������λС������
���� ��1����ѧ����ʽ��ѭԭ�Ӹ����غ㣬����ԭ�Ӹ����غ��ж�ȱ�����ʣ�
��2����������������ڽӴ��ҷ���������Ӧ������������ӦΪ���淴Ӧ��
��3��a��������������������������ĽӴ������
b�����淴Ӧ�е���Ӧ���ж��֣�����һ�ַ�Ӧ��Ũ�ȿ������������Ӧ��ת���ʣ�
c��������ͬ�ȳ̶ȸı䷴Ӧ���ʣ�ƽ�ⲻ�ƶ���
d�����ݷ�Ӧԭ����֪����¯�ų��Ŀ���������������
��4��ÿ160g SO3������H2O��l�����Ϸų�260.6kJ��������80g����������ˮ��Ӧ����130.3KJ�������Ȼ�ѧ����ʽ����д����д����
��5���ٶ������������嵥�����õ��Ƕ�������Ļ�ԭ�Ժ��嵥�ʵ������ԣ�����������ԭ��Ӧ��д���ӷ���ʽ��
�ڱ���Ӧ�����ǣ�OH-�����Ǻ�NH4HSO3�е�H+��Ӧ������ж��OH-�ٺ�NH4+��Ӧ�ų�����������������ε���������NH4HSO3����Ҳ���ų��İ���������Ϊ0��Ũ�������ӵ��������ǰ�������������һ�κ͵ڶ��ηų��İ���������һ���ģ�����˵��һ�ο϶���OH-�������������õڶ��ε������㣨��Ϊ��OH-���㣩��
��� �⣺��1������ԭ�Ӹ����غ��֪ȱ������ΪFeS2��
�ʴ�Ϊ��FeS2��
��2�����������������ڽӴ����ڷ���������ԭ��Ӧ������������ӦΪ���淴Ӧ������ʽ��2SO2+O2$?_{���¸�ѹ}^{����}$2SO3��
�ʴ�Ϊ��2SO2+O2$?_{���¸�ѹ}^{����}$2SO3��
��3��a��������������������������ĽӴ���������Խ���������飬���Գ��ȼ�գ���a��ȷ��
b�����ݷ���ʽ��2SO2+O2$?_{���¸�ѹ}^{����}$2SO3����֪��Ӧ���������Ͷ����������֣���������������Ũ�ȿ�����߶��������ת���ʣ���b��ȷ��
c��������ʹ�öԻ�ѧƽ�ⲻ����Ӱ�죬��������ת���ʲ��䣬��c����
d�����ݷ�Ӧԭ����֪����¯�ų��Ŀ�������������������������������d��ȷ��
��ѡ��abd��
��4��ÿ160g SO3������H2O��l�����Ϸų�260.6kJ��������80g����������ˮ��Ӧ����130.3KJ����Ӧ���Ȼ�ѧ����ʽΪ��SO3��g��+H2O��l��=H2SO4��l������H=-130.3kJ/mol��
�ʴ�Ϊ��SO3��g��+H2O��l��=H2SO4��l������H=-130.3kJ/mol��
��5����SO2����Br2�ķ�Ӧ�ж�����������Ϊ���ᣬ�嵥�ʱ���ԭΪ�廯�⣬���ӷ���ʽΪSO2+Br2+2H2O=4H++2Br-+SO42-��
�ʴ�Ϊ��SO2+Br2+2H2O=4H++2Br-+SO42-��
�ڵõ�����β�Ʒ�ǣ�NH4��2SO3��NH4HSO3�Ļ�������Ӧ�����ǣ�OH-�����Ǻ�NH4HSO3�е�H+��Ӧ������ж��OH-�ٺ�NH4+��Ӧ�ų�����������������ε���������NH4HSO3����Ҳ���ų��İ���������Ϊ0��
����֪�������Ϊ30.00gʱ������0.04molNH3���������NH4HSO4����NaOH��Һ��Ӧ��2NH4HSO4+2NaOH=��NH4��2SO4+Na2SO4+H2O��ֻ�е�NH4HSO4�е�H+������ȫ��NH4+������NaOH��Һ��Ӧ����NH3��NH4++OH-=NH3��+H2O���ݴ��ж��������Ϊ10.00gʱNaOH��Һ�������������Ϊ20.00g��30.00gʱ�����ĵ�NaOH������ȣ���10.00g�����NH4HSO4 �루NH4��2SO4�����ʵ����ֱ�ΪX��Y��n��NH3��=n��OH-��-n��H+�������У�
| �������/g | 10.00 | 20.00 | 30.00 | 40.00 |
| ��NH4HSO4����NH4��2SO4/mol | X��Y | 2X��2Y | 3X��3Y | 4X��4Y |
| ����NH3/mol | X+2Y | X+2Y | 0.04 | 0 |
| ����NaOH/mol | 2X+2Y | 3X+2Y | 3X+0.04 | 3X+0.04 |
��15.00 g�����NaOH��Һ��Ӧ������NH3��֪Ũ�������ӵ����������������ۿ�֪��NaOH��Һ�й���0.232molNaOH�����������Ϊ15.00gʱ��0.096mol NH4HSO4��0.03mol ��NH4��2SO4������NH4+��H+ 0.252mol����NaOH���㣬��ʱ����n��NH3��=��0.232-0.096��mol=0.136mol��NH3������=0.136mol��17g/mol=2.31g��Ũ�������ӵ�����Ϊ2.31g��
�ʴ�Ϊ��14.56�� 2.31 g��
���� ���⿼���˹�ҵ������Ļ���ԭ�������黯ѧƽ���Ӱ�����غͻ�ѧ��Ӧ���ʵ�Ӱ�����أ������Ṥҵβ��������Ϊ���忼�黯ѧ���㣬�漰�������㣬��������ļ��㡢��Χ�����ͼ��㡢��ϢǨ���ͼ��㡢NH4+��H+��NaOH��Һ��Ӧ���Ⱥ�˳���֪ʶ�������붨�����ϣ��ۺ���ǿ����Ŀ�ѶȽϴ�

 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�| A�� | ���Ӵ�������H2SO4ʱ���������εķ�Ӧԭ��Ϊ��2SO2��g��+O2��g��$?_{��}^{����}$2SO3��g����H��0 | |
| B�� | ��ˮ��þ����Ҫ����Ϊ����ˮ$\stackrel{CaCO_{3}��s��}{��}$Mg��OH��2��s��$\stackrel{����}{��}$MgCl2��aq��$\stackrel{���}{��}$Mg��l��+Cl2��g�� | |
| C�� | ��ͨˮ�����Ҫ�ɷ��ǹ���� | |
| D�� | �������Ҫ�ɷ��������������� |
| A�� | ʹ�þ۶�����̼���ϻ������ɫ��Ⱦ | |
| B�� | �۶�����̼������ͨ���ۺϷ�Ӧ�Ƶõ� | |
| C�� | �۶�����̼������ɱ���Ϊͬ���칹�� | |
| D�� | �۶�����̼�ܸɱ������ڴ����� |
| ʵ����� | ʵ������ | ���� | |
| A | �ñ���̼������Һ���ݹ�¯��������ˣ�ϴ�ӣ������ó��������ټ���ϡ���� | �����ݲ��� | �ɳ�ȥ��¯�������е�CaSO4 |
| B | ij����NO2���ܱ������У�����Ӧƽ������¶Ȳ��䣬����������� | ������ɫ��dz | ƽ��2NO2��g��?N2O4��g�������ƶ� |
| C | ��Ʒ����Һ��ͨ��ij���� | ��Һ��ɫ | ��������SO2 |
| D | �ýྻ��˿պȡ��Һ���ھƾ��ƻ��������� | ����ʻ�ɫ | ��Һ�к�Na+����K+ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �ü�������������Һ����������Һ���ܲ��������ġ�ͨ·�����ǵ�����Һ | |
| B�� | ��ij����ͨ����۵⻯����Һ�У���Һ����ɫ��������һ����Cl2 | |
| C�� | ��ij��Һ�м���AgNO3��Һ��������ɫ����������Һ��һ����Cl- | |
| D�� | ��ijϡ��Һ�м�������NaOH��Һ��δ����ʹʪ��ĺ�ɫʯ����ֽ���������壬����Һ��һ�� ����NH4+ |
 ����ϩ��ˮ�����д��칦�ܣ�
����ϩ��ˮ�����д��칦�ܣ�
