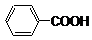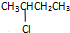��Ŀ����
A��B��C��D�����ֶ�����Ԫ�أ�E�ǹ���Ԫ�أ�A��B��Cͬ���ڣ�C��Dͬ���壬A��ԭ�ӽṹʾ��ͼΪ��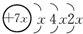 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C�������������δ�ɶԵ��ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C�������������δ�ɶԵ��ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
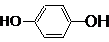
��1���û�ѧʽ��ʾ��������Ԫ��������������Ӧ��ˮ������������ǿ���� ��������ǿ���� �����ѧʽ��
��2����Ԫ�ط��ű�ʾD�������ڵ�һ����������Ԫ���� ���縺������Ԫ���� ��
��3��EԪ�������ڱ���λ�� ����֪Ԫ�����ڱ��ɰ������Ų���Ϊs����p���ȣ���EԪ���� ����
��4������D�ĵ����Ų�ͼ �������Ų���ѭ�� ԭ���� ����
��5��C���⻯��Ϊ ���ӣ����Ի�Ǽ��ԣ�
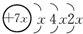 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C�������������δ�ɶԵ��ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C�������������δ�ɶԵ��ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺��1���û�ѧʽ��ʾ��������Ԫ��������������Ӧ��ˮ������������ǿ����
��2����Ԫ�ط��ű�ʾD�������ڵ�һ����������Ԫ����
��3��EԪ�������ڱ���λ��
��4������D�ĵ����Ų�ͼ
��5��C���⻯��Ϊ
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������A��B��C��D�����ֶ�����Ԫ�أ�A��ԭ�ӽṹʾ��ͼΪ��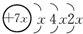 ��K���������2�����ӣ�����x=2����Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ�
��K���������2�����ӣ�����x=2����Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ�
A��B��Cͬ���ڣ�B��ͬ���ڵ�һ��������С��Ԫ�أ���B��NaԪ�أ�C�������������δ�ɶԵ��ӣ���CΪPԪ�أ�
C��Dͬ���壬��D���ڶ�����Ԫ�أ���DΪNԪ�أ�
E�ǹ���Ԫ�أ�E����Χ�����Ų�ʽΪ3d64s2��E���������=2+8+8+6+2=26��ΪFeԪ�أ�
��1��Ԫ�صķǽ�����Խǿ��������������Ӧ��ˮ����������Խǿ��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ��
��2��ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
Ԫ�صķǽ�����Խǿ����縺��Խǿ��
��3��E��FeԪ�أ�FeԪ�������ڱ���λ���ǵ������ڵ�VIII�壬��VIII��Ԫ������d����
��4��D��NԪ�أ����������Ų�ʽΪ1s22s22p3�����������Ų�Ҫ��ѭ����ԭ�������ع���
��5��C��PԪ�أ�P���⻯��ΪPH3��������������������غϵ�Ϊ�Ǽ��Է��ӣ�����Ϊ���Է��ӣ�
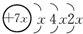 ��K���������2�����ӣ�����x=2����Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ�
��K���������2�����ӣ�����x=2����Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ�A��B��Cͬ���ڣ�B��ͬ���ڵ�һ��������С��Ԫ�أ���B��NaԪ�أ�C�������������δ�ɶԵ��ӣ���CΪPԪ�أ�
C��Dͬ���壬��D���ڶ�����Ԫ�أ���DΪNԪ�أ�
E�ǹ���Ԫ�أ�E����Χ�����Ų�ʽΪ3d64s2��E���������=2+8+8+6+2=26��ΪFeԪ�أ�
��1��Ԫ�صķǽ�����Խǿ��������������Ӧ��ˮ����������Խǿ��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ��
��2��ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
Ԫ�صķǽ�����Խǿ����縺��Խǿ��
��3��E��FeԪ�أ�FeԪ�������ڱ���λ���ǵ������ڵ�VIII�壬��VIII��Ԫ������d����
��4��D��NԪ�أ����������Ų�ʽΪ1s22s22p3�����������Ų�Ҫ��ѭ����ԭ�������ع���
��5��C��PԪ�أ�P���⻯��ΪPH3��������������������غϵ�Ϊ�Ǽ��Է��ӣ�����Ϊ���Է��ӣ�
���
�⣺A��B��C��D�����ֶ�����Ԫ�أ�A��ԭ�ӽṹʾ��ͼΪ��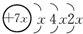 ��K���������2�����ӣ�����x=2����Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ�
��K���������2�����ӣ�����x=2����Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ�
A��B��Cͬ���ڣ�B��ͬ���ڵ�һ��������С��Ԫ�أ���B��NaԪ�أ�C�������������δ�ɶԵ��ӣ���CΪPԪ�أ�
C��Dͬ���壬��D���ڶ�����Ԫ�أ���DΪNԪ�أ�
E�ǹ���Ԫ�أ�E����Χ�����Ų�ʽΪ3d64s2��E���������=2+8+8+6+2=26��ΪFeԪ�أ�
��1��Ԫ�صķǽ�����Խǿ��������������Ӧ��ˮ����������Խǿ��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ���⼸��Ԫ���зǽ�������ǿ����NԪ�أ�����HNO3��������ǿ����������ǿ����Na������NaOH�ļ�����ǿ���ʴ�Ϊ��HNO3��NaOH��
��2��ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�Ԫ�صķǽ�����Խǿ����縺��Խǿ��D��NԪ�أ�λ�ڵڶ����ڣ��ڶ����ڵ�һ������������NeԪ�أ��縺��������FԪ�أ��ʴ�Ϊ��Ne��F��
��3��E��FeԪ�أ�FeԪ�������ڱ���λ���ǵ������ڵ�VIII�壬��VIII��Ԫ������d����
�ʴ�Ϊ���������ڵ�VIII�壻d��
��4��D��NԪ�أ����������Ų�ʽΪ1s22s22p3�����������Ų�ͼΪ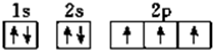 �����������Ų�Ҫ��ѭ����ԭ�������ع���
�����������Ų�Ҫ��ѭ����ԭ�������ع���
�ʴ�Ϊ�� �����������أ�
�����������أ�
��5��C��PԪ�أ�P���⻯��ΪPH3���÷���Ϊ�����νṹ������������������IJ��غϣ�����Ϊ���Է��ӣ��ʴ�Ϊ�����Է��ӣ�
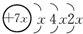 ��K���������2�����ӣ�����x=2����Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ�
��K���������2�����ӣ�����x=2����Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ�A��B��Cͬ���ڣ�B��ͬ���ڵ�һ��������С��Ԫ�أ���B��NaԪ�أ�C�������������δ�ɶԵ��ӣ���CΪPԪ�أ�
C��Dͬ���壬��D���ڶ�����Ԫ�أ���DΪNԪ�أ�
E�ǹ���Ԫ�أ�E����Χ�����Ų�ʽΪ3d64s2��E���������=2+8+8+6+2=26��ΪFeԪ�أ�
��1��Ԫ�صķǽ�����Խǿ��������������Ӧ��ˮ����������Խǿ��Ԫ�صĽ�����Խǿ��������������ˮ�������Խǿ���⼸��Ԫ���зǽ�������ǿ����NԪ�أ�����HNO3��������ǿ����������ǿ����Na������NaOH�ļ�����ǿ���ʴ�Ϊ��HNO3��NaOH��
��2��ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�Ԫ�صķǽ�����Խǿ����縺��Խǿ��D��NԪ�أ�λ�ڵڶ����ڣ��ڶ����ڵ�һ������������NeԪ�أ��縺��������FԪ�أ��ʴ�Ϊ��Ne��F��
��3��E��FeԪ�أ�FeԪ�������ڱ���λ���ǵ������ڵ�VIII�壬��VIII��Ԫ������d����
�ʴ�Ϊ���������ڵ�VIII�壻d��
��4��D��NԪ�أ����������Ų�ʽΪ1s22s22p3�����������Ų�ͼΪ
 �����������Ų�Ҫ��ѭ����ԭ�������ع���
�����������Ų�Ҫ��ѭ����ԭ�������ع����ʴ�Ϊ��
 �����������أ�
�����������أ���5��C��PԪ�أ�P���⻯��ΪPH3���÷���Ϊ�����νṹ������������������IJ��غϣ�����Ϊ���Է��ӣ��ʴ�Ϊ�����Է��ӣ�
���������⿼��λ�ýṹ���ʵ����ϵ��Ӧ�ã��漰���Ӽ����жϡ���������Ų���Ԫ��λ���жϡ�Ԫ�������ɵ�֪ʶ�㣬���ؿ���֪ʶ�����������ѵ��Ǻ�������Ų�ʽ����д��֪ʶ��������ã���Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
����ʵ������������ǣ�������
| A���к͵ζ�ʵ���е���ƿʹ��ǰӦ���ô�װҺϴ�� |
| B����25 mL�ζ��ܽ��еζ�ʵ��ʱ������ij��Һ���Ϊ21.70 mL |
| C����������ƽ�������ϸ���һ�Žྻ��ֽƬֱ�ӳ���NaOH���� |
| D���ù㷺pH��ֽ���ij��Һ��pHΪ2.3 |
һ���¶��£���2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��仯��������ͼ��ʾ������������ȷ���ǣ�������


| A����Ӧ��ʼ��10s����Z��ʾ�ķ�Ӧ����Ϊ0.158mol/��L?s�� |
| B����Ӧ��ʼ��10s��X�����ʵ���Ũ�ȼ�����0.79mol/L |
| C����Ӧ��ʼ��10sʱ��Yת����0.79mol |
| D����Ӧ�Ļ�ѧ����ʽΪ��X��g��+Y��g��?Z��g�� |
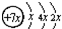 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺