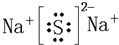��Ŀ����
�������ж�����Ԫ�����ʵ�����

�ش���������
��1��Ԫ�آ������ڱ���λ����_____________��Ԫ�آ���Ԫ�آ���Ƚϣ���̬�⻯����ȶ�����
_________���ѧʽ����
��2��Ԫ�آ���_________��дԪ�ط��ţ���Ԫ�آ���_________��дԪ�ط��ţ������߰���ԭ�Ӹ�����Ϊ1��1�γɵĻ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ___________________________��
��3��Ԫ�آ���Ԫ�آڵķǽ�����ǿ��˳��Ϊ_______________��дԪ�ط��ţ���Ԫ�آݵĵ��ʼ��뵽Ԫ�آڵ��⻯���ˮ��Һ�У���Ӧ�Ļ�ѧ����ʽΪ__________________________��
��4���õ���ʽ��ʾԪ�آ��⻯����γɹ���_________________________��д��Ԫ�آ��⻯��ĵ���ʽ
__________________��д��ʵ������Ԫ�آݵ��ʵĻ�ѧ��Ӧ����ʽ___________________________��
��1��Ԫ�آ������ڱ���λ����_____________��Ԫ�آ���Ԫ�آ���Ƚϣ���̬�⻯����ȶ�����
_________���ѧʽ����
��2��Ԫ�آ���_________��дԪ�ط��ţ���Ԫ�آ���_________��дԪ�ط��ţ������߰���ԭ�Ӹ�����Ϊ1��1�γɵĻ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ___________________________��
��3��Ԫ�آ���Ԫ�آڵķǽ�����ǿ��˳��Ϊ_______________��дԪ�ط��ţ���Ԫ�آݵĵ��ʼ��뵽Ԫ�آڵ��⻯���ˮ��Һ�У���Ӧ�Ļ�ѧ����ʽΪ__________________________��
��4���õ���ʽ��ʾԪ�آ��⻯����γɹ���_________________________��д��Ԫ�آ��⻯��ĵ���ʽ
__________________��д��ʵ������Ԫ�آݵ��ʵĻ�ѧ��Ӧ����ʽ___________________________��
��1���ڶ�����IA�壻NH3
��2��O��Na��2Na2O2+2H2O==4NaOH+O2��
��3��Cl��S��Cl2+H2S==2HCl+S��
��4�����ԡ�
��2��O��Na��2Na2O2+2H2O==4NaOH+O2��
��3��Cl��S��Cl2+H2S==2HCl+S��
��4�����ԡ�

��ϰ��ϵ�д�
�����Ŀ