��Ŀ����
7�� �����������ֲ�ʹ��п�����й�������ĩ�꡶�칤���һ����������������Ĺ�����п�����ļ��أ��ش��������}��
�����������ֲ�ʹ��п�����й�������ĩ�꡶�칤���һ����������������Ĺ�����п�����ļ��أ��ش��������}����1����̬Znԭ�ӵĵ���ռ��������ߵ��ܲ����ΪN����Znͬ���ڵ����и���Ԫ�صĻ�̬ԭ���У�������������Zn��ͬ��Ԫ����4�֣�
��2������п���ڹ����İ�ˮ���γ�[Zn��NH3��4]SO4��Һ��
����[Zn��NH3��4]SO4�У������ӵ�����ԭ�ӵĹ���ӻ�����Ϊsp3��
����[Zn��NH3��4]SO4�У���Zn2+�γ���λ����ԭ���ǵ�����Ԫ�����ƣ���
��д��һ����SO42-��Ϊ�ȵ�����ķ��ӵĻ�ѧʽ$P{O}_{4}^{3-}$��
��NH3��������ˮ������Ϊ���Ƕ��Ǽ��Է����⣬����ΪNH3��ˮ�����γɷ��Ӽ�������ܽ������
��3��Zn��S���γɻ����ᄃ��ľ�����ͼ��ʾ��
����Znԭ�Ӿ��������Znԭ����12����
�ڸû�����Ļ�ѧʽΪZnS��
����֪�þ���ľ�������a=541pm�����ܶ�Ϊ$\frac{4��97}{��541��1{0}^{-10}��^{3}{N}_{A}}$g•cm-3���г�����ʽ���ɣ���
���� ��1��Zn�����ڱ��е�λ���ǵ������ڣ���IIB�壬�����ĸ��ܲ㣬��Znͬ���ڵ����и���Ԫ���У�����������Zn��ͬ����Sc��3d14s2����Ti��3d24s2����V��3d34s2����Mn��3d54s2����
��2������[Zn��NH3��4]SO4�У�������Ϊ$S{O}_{4}^{2-}$������VSEPR���ۺ��ӻ���������ж��ӻ����ͣ�
����[Zn��NH3��4]SO4�У�������NH3����Zn2+�γ���λ������N��
�۵ȵ�������ָ��ԭ������ͬ�£�ԭ�ӵļ۵�������ͬ�����ӣ�ͨ������Ԫ����������ƽ�Ʒ���ͬʱ����������ȷ���ȵ��������ӣ�
��NH3������ˮ�����γɷ��Ӽ�����������ܽ�ȣ�
��3�����뵽ZnS��������ʯ�����������ԣ�
��ע�������Ӧ�ǹ�����һ������Χ�����ྦྷ�����ӵ��Ŀ�Zn2+����֮�Ⱦ������Zn2+��12����
�ڼ��㾧���е�������������������������������ռ$\frac{1}{8}$����������ռ$\frac{1}{2}$�������ڲ�ԭ��Ϊ�����������У��ݴ˽��
�۸��ݾ������ܶȼ��㹫ʽ��$��=\frac{z{M}_{r}}{{N}_{A}V}$������zΪ�����ڲ���������MrΪ���ӵ����������VΪһ�����������
��� �⣺��1��Znλ��Ԫ�����ڱ��е�λ���ǵ������ڣ���IIB�壬�����ĸ��ܲ㣬ΪK��L��M��N������ܲ�ΪN������Ҳ��ߣ�Zn�������ΪN�㣬��������Ϊ4s2����Zn������ͬ���ڣ���������������ҲΪ4s2�ĸ���Ԫ����Sc��3d14s2����Ti��3d24s2����V��3d34s2����Mn��3d54s2������ע�⣬�ڰ��岻���ڸ����ڣ���˹���4�֣�
�ʴ�Ϊ��N��4��
��2������[Zn��NH3��4]SO4�У�������Ϊ$S{O}_{4}^{2-}$������VSEPR���ۣ�$S{O}_{4}^{2-}$�гɼ�ԭ����ΪBP=4���µ��Ӷ���Ϊ$LP=\frac{6-2��4+2}{2}=0$������۵��Ӷ���ΪVP=BP+LP=4+0=4�������ӻ�������ۣ�����ԭ��S���ӻ�����Ϊsp3�ӻ����ʴ�Ϊ��sp3��
����[Zn��NH3��4]SO4�У�����ΪNH3������N�Ϻ��й¶Ե��ӣ�Zn2+�ṩ�չ�����Ӷ��γ���λ�������������Zn2+�γ���λ����ΪNԭ�ӣ�
�ʴ�Ϊ������
�۵ȵ�������ָ��ԭ������ͬ�£�ԭ�ӵļ۵�������ͬ�����ӣ�ͨ������Ԫ����������ƽ�Ʒ���ͬʱ����������ȷ���ȵ��������ӣ���ˣ���$S{O}_{4}^{2-}$��Ϊ�ȵ��������$P{O}_{4}^{3-}$��$Cl{O}_{4}^{-}$��CCl4�ȣ��ʴ�Ϊ��$P{O}_{4}^{3-}$����$Cl{O}_{4}^{-}$��CCl4����
��NH3��������ˮ������Ϊ���Ƕ��Ǽ��Է��ӣ�NH3������ˮ�����γɷ��Ӽ�����������ܽ�ȣ�
�ʴ�Ϊ��NH3��ˮ�����γɷ��Ӽ�������ܽ������
��3��ZnS�ľ�������ʯ�ľ������ƣ�
�پ�����Ӧ��Ϊһ�����������ڣ���Χ�����ྦྷ����֮��������ZnS�����ĵ��Ŀ�Zn2+���������滹��һ��������֮������������ĵ�Zn2+�Ⱦ��������Zn2+��12�����ʴ�Ϊ��12��
�ڶ���������������������ռ$\frac{1}{8}$����������ռ$\frac{1}{4}$�������ڲ�ԭ��Ϊ�����������У���һ��ZnS�����У�Zn2+����Ϊ$8��\frac{1}{8}+6��\frac{1}{2}=4$��S2-����Ϊ4����˸û����ﻯѧʽΪZnS��
�ʴ�Ϊ��ZnS��
��ȡ1mol����������NA����������֪��������Ϊa=541pm����һ�����������Ϊ${V}_{0}=��541��1{0}^{-10}��^{3}c{m}^{3}$��1molZnS�����У���4molZn2+��4molS2-����һ������������Ϊm=4mol��65g/mol+4mol��32g/mol=4��97g����˾������ܶ�Ϊ$��=\frac{m}{{N}_{A}{V}_{0}}=\frac{4��97}{��541��1{0}^{-10}��^{3}{N}_{A}}g/c{m}^{3}$��
�ʴ�Ϊ��$\frac{4��97}{��541��1{0}^{-10}��^{3}{N}_{A}}$��
���� ���⿼�����ʽṹ��֪ʶ��Ԫ�����ڱ���ԭ�ӹ�����۲���ӶԻ������ۣ��ӻ���������ж����ӵĿռ乹�ͣ�������γɣ������ļ����֪ʶ�㣬��Ŀ���ۺϣ��ѶȲ����ǻ����⣮

| A�� | N2 | B�� | HCl | C�� | MgCl2 | D�� | Na2O2 |
| A�� | ʵ����������FeCl3��Һʱ�������м�������ϡ���� | |
| B�� | �����ڳ�ʪ�Ļ����������� | |
| C�� | Mg��OH��2�������ܽ���NH4Cl��Һ�� | |
| D�� | �ȵĴ�����Һȥ����Ч���� |

| A�� | X�������Ҵ� | |
| B�� | ���е���������������X��ˮ�� | |
| C�� | X��ȫȼ�պ�����CO2��H2O�����ʵ�����Ϊ1��1 | |
| D�� | ����ʽΪ C5H10O2������NaHCO3��Һ��Ӧ���������������5�� |
| ѡ�� | ʵ����� | ���� | ���� |
| A | �ⶨ����ʱ�ı���HCOONa��Һ��CH3COONa��Һ��pH | HCOONa��pH��CH3COONa��pH | ��������ԣ�HCOOH��CH3COOH |
| B | ��5ml 2mol•L-1NaOH��Һ�м���1ml 1mol•L-1CuSO4��Һ�������0.5ml�л���X������ | δ����ש��ɫ���� | ˵��X�в�����ȩ�� |
| C | KBrO3��Һ�м�����������Ȼ��ͨ������Cl2 | �л���ʳȺ�ɫ | �����ԣ�Cl2��Br2 |
| D | NaAlO2��Һ��NaHCO3��Һ��� | �а�ɫ��״�������� | ����ˮ����ٽ��������������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
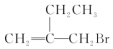
| A�� | �����ʿ��Է���ˮ�ⷴӦ | |
| B�� | �������ܺ�AgNO3��Һ��Ӧ����AgBr���� | |
| C�� | �����ʿ��Է�����ȥ��Ӧ | |
| D�� | �����ʲ���ʹ������Ȼ�̼��Һ��ɫ |


 �ĺϳ�·�ߣ�
�ĺϳ�·�ߣ� ��
��