��Ŀ����
�±���Ԫ�����ڱ��е�һ���֣��ش������й����⣺
��1����D��E��F��G����Ԫ���γɵ����ӣ��뾶�ɴ�С��˳����
����Ԫ�ط��Ż�ѧʽ����ͬ����
��2����Ԫ��E�ĵ�����ˮ��Ӧ�����ӷ���ʽΪ ��Ԫ��B�ĵ�����ˮ��Ӧ�����ӷ���ʽΪ ��
��3����B������������Ӧˮ������C�����������Ӧ�����ӷ���ʽ ��
��4����Ԫ��A�ĵ��ʵĽṹʽΪ ��Ԫ��H���������ĵ���ʽ ��
| �� ���� |
IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| 2 | H | A | ||||||
| 3 | B | C | D | E | ||||
| 4 | F | G |
����Ԫ�ط��Ż�ѧʽ����ͬ����
��2����Ԫ��E�ĵ�����ˮ��Ӧ�����ӷ���ʽΪ
��3����B������������Ӧˮ������C�����������Ӧ�����ӷ���ʽ
��4����Ԫ��A�ĵ��ʵĽṹʽΪ
���㣺Ԫ�����ڱ��Ľṹ����Ӧ��,Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
����������Ԫ�������ڱ��е�λ��֪��A��B��C��D��E��F��G��H�ֱ���N��Na��Al��S��Cl��K��Ca��CԪ�أ�
��1�����Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ�������������С��
��2��������ˮ��Ӧ��������ʹ����ᣬ�ƺ�ˮ��Ӧ�����������ƺ�������
��3����������ǿ�Ӧ����ƫ�����κ�ˮ��
��4������������������ԭ��֮����ڹ���������������̼������ÿ����ԭ�Ӻ�̼ԭ���γ��������Ӷԣ�
��1�����Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ�������������С��
��2��������ˮ��Ӧ��������ʹ����ᣬ�ƺ�ˮ��Ӧ�����������ƺ�������
��3����������ǿ�Ӧ����ƫ�����κ�ˮ��
��4������������������ԭ��֮����ڹ���������������̼������ÿ����ԭ�Ӻ�̼ԭ���γ��������Ӷԣ�
���
�⣺����Ԫ�������ڱ��е�λ��֪��A��B��C��D��E��F��G��H�ֱ���N��Na��Al��S��Cl��K��Ca��CԪ�أ�
��1�����������ӵ��Ӳ�ṹ��ͬ�������Ӱ뾶����ԭ���������������С���������Ӱ뾶��С˳����S2-��Cl-��K+��Ca2+���ʴ�Ϊ��S2-��Cl-��K+��Ca2+��
��2��E�ĵ�����������B�ĵ������ƣ�������ˮ��Ӧ��������ʹ����ᣬ���ӷ���ʽΪCl2+H2O=Cl-+H++HClO���ƺ�ˮ��Ӧ�����������ƺ����������ӷ���ʽΪ2Na+2H2O=2Na++2OH-+H2����
�ʴ�Ϊ��Cl2+H2O=Cl-+H++HClO��2Na+2H2O=2Na++2OH-+H2����
��3��B������������ˮ������NaOH��C���������������������߷�Ӧ����ƫ�����ƺ�ˮ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
��4��A��NԪ�أ�����������������ԭ��֮����ڹ�����������ṹʽΪN��N��������̼������ÿ����ԭ�Ӻ�̼ԭ���γ��������Ӷԣ������ʽΪ ���ʴ�Ϊ��N��N��
���ʴ�Ϊ��N��N�� ��
��
��1�����������ӵ��Ӳ�ṹ��ͬ�������Ӱ뾶����ԭ���������������С���������Ӱ뾶��С˳����S2-��Cl-��K+��Ca2+���ʴ�Ϊ��S2-��Cl-��K+��Ca2+��
��2��E�ĵ�����������B�ĵ������ƣ�������ˮ��Ӧ��������ʹ����ᣬ���ӷ���ʽΪCl2+H2O=Cl-+H++HClO���ƺ�ˮ��Ӧ�����������ƺ����������ӷ���ʽΪ2Na+2H2O=2Na++2OH-+H2����
�ʴ�Ϊ��Cl2+H2O=Cl-+H++HClO��2Na+2H2O=2Na++2OH-+H2����
��3��B������������ˮ������NaOH��C���������������������߷�Ӧ����ƫ�����ƺ�ˮ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
��4��A��NԪ�أ�����������������ԭ��֮����ڹ�����������ṹʽΪN��N��������̼������ÿ����ԭ�Ӻ�̼ԭ���γ��������Ӷԣ������ʽΪ
 ���ʴ�Ϊ��N��N��
���ʴ�Ϊ��N��N�� ��
��
���������⿼����Ԫ�����ڱ���Ԫ�������ɵ��ۺ�Ӧ�ã�����Ԫ�������ڱ��е�����ȷ��Ԫ�أ��ٽ�����ʵ����ʡ�Ԫ�������������������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
�����Ŀ
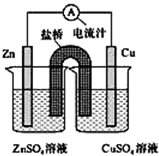 ��ͼΪһԭ��ص�װ��ʾ��ͼ������˵������ȷ���ǣ�������
��ͼΪһԭ��ص�װ��ʾ��ͼ������˵������ȷ���ǣ�������| A��ԭ��ع���ʱ���ܷ�ӦΪ��Zn+Cu2+=Zn2++Cu |
| B��ԭ��ع���ʱ��������ͭ����������������п�� |
| C��ԭ��ع���ʱ��������K+����CuSO4��Һ |
| D�������Cu�缫��Zn�缫������������ָ�뷴��ƫת |
����˵������ȷ���ǣ�������
| A��2mol CH4��������O2��Ħ����������32g |
| B��1mol�κ������ж�������ͬ��ԭ���� |
| C��0.5molNaClԼ����6.02��1023������ |
| D��1mol/LKCl��Һ�к�������1mol |



