��Ŀ����
1�� ����ϩ��ʯ���ѽ�������Ҫ�ɷ֣���ϩ�Ľṹ��ʽΪH2C=CH2��
����ϩ��ʯ���ѽ�������Ҫ�ɷ֣���ϩ�Ľṹ��ʽΪH2C=CH2����ʵ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
��1��д����ȡ���������Ļ�ѧ��Ӧ����ʽCH3COOH+C2H5OH$?_{��}^{Ũ����}$ CH3COOC2H5+H2O���÷�Ӧ������������Ӧ��
��2������̼������Һ����Ҫ�����������Ҵ������ἰ���������������ܽ�ȣ�
��3����Ҫ���Ƶõ������������������Ӧ���õ�ʵ������Ƿ�Һ����д�������ƣ���
��4��ʵ��ʱ����ʢ������Ҵ����Թ�����뼸�����Ƭ���������Ƿ�ֹ���У�
���� ����ϩ�Ǻ���̼̼˫�������ϩ����
��1��������Ҵ���Ũ���������·���������Ӧ����������������ˮ������������Ӧ��
��2���Ʊ���������ʱ���ñ���̼������Һ��������������Ŀ���dz�ȥ�Ҵ������ᡢ���������������ܽ�ȣ����ڷֲ㣻
��3���������������ڱ���̼������Һ��
��4�����Ƭ�ɷ�ֹ���У�
��� �⣺����ϩ�к�̼��̼֮���Թ���˫����ϣ��ṹ��ʽΪ��H2C=CH2���ʴ�Ϊ��H2C=CH2��
��1���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��ͬʱ�÷�Ӧ���棬��Ӧ�Ļ�ѧ����ʽΪCH3COOH+C2H5OH $?_{��}^{Ũ����}$CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+C2H5OH $?_{��}^{Ũ����}$ CH3COOC2H5+H2O��������Ӧ��
��2���Ʊ���������ʱ���ñ���̼������Һ����������������ȥ�Ҵ������ᡢ���������������ܽ�ȣ����ڷֲ㣮�ʴ�Ϊ�����������������ܽ�ȣ�
��3���������������ڱ���̼������Һ�����÷�Һ�ķ������룬�ʴ�Ϊ����Һ��
��4����Һ������������ױ��У��������Ƭ���ɷ�ֹ���У��ʴ�Ϊ����ֹ���У�
���� ���⿼�����Ʊ���������ƣ���Ŀ�ѶȲ�����ȷ������Ӧԭ��Ϊ���ؼ���ע������������Ӧ��Ũ���ᡢ����̼������Һ��Һ�����ã�����������ѧ���ķ�����������ѧʵ��������

 ��У����ϵ�д�
��У����ϵ�д�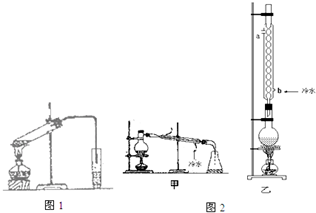 ��֪������ݣ�
��֪������ݣ�| �� �� | 2��4��6 �۵�/�� | �е�/�� | �ܶ�/g•cm-3 |
| �� �� | -114 | 78 | 0.789 |
| �� �� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.900 |
| ŨH2SO4 | 338 | 1.84 |
��ͬѧ�ǻش��������⣺
��1��������У�������һ�����Ļ��Һ�IJ������ȼ�������Ҵ���4mL���ٻ�������1mLŨH2SO4���ӱ���
��2��д���÷�Ӧ�Ļ�ѧ����ʽCH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOC2H5+H2O��ŨH2SO4�������Ǵ�������ˮ����
��3��������У���С������Թ��еĻ��Һ����ԭ����������ᡢ�Ҵ������������е�ӽ��ҽϵͣ������ȣ���Ӧ�����������ʧ��
��4����������۲쵽����������dz��ɫNa2CO3��Һ�ϲ���Լ4cm�����ɫҺ�壬��Na2CO3��Һ���ɫ��dz�������ݣ��ϲ�Һ��䱡��д��ԭ�����ϲ����Ͳ���Ϊ���ɵ���������������ˮ�����ܶȱ�ˮС��ͬʱ��Ϊ�ӷ�������������̼���Ʒ�Ӧ���ų�CO2���壬���������ݳ���
��5��������У��������������ѡ�õ������Ƿ�Һ©��������Ӧ���Ͽڵ�������Ϊ����������ˮ�ܶ�С��
��6��Ϊ������������IJ��ʣ��ס�����λͬѧ�ֱ��������ͼ2�ס��ҵ�װ�ã���ͬѧ����Ӧ�����ȴ�����ñ���Na2CO3��Һ��ȡ��ƿ�в��������Ϊ��װ�ú�������Ϊ��Ӧ��������������
2NH3��g��+CO2��g���TNH2COONH4��s����H��0
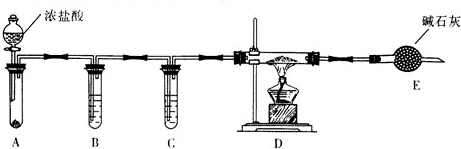
��1��������ͼIװ����ȡ��������ƿ�п�ѡ����Լ����������ƹ��壨��Ũ��ˮ���ʯ�һ�Ũ��ˮ����ʯ�ң��ȣ�
��2���Ʊ���������淋�װ������ͼ����ʾ����NH3��CO2ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ�������淋�С����������CCl4�У���������϶�ʱ��ֹͣ�Ʊ���

ע��CCl4��Һ��ʯ����Ϊ���Խ��ʣ�
��ͼI�еμ�Һ������������Ƿ�Һ©����Һ��ʯ������ƿ��������ͨ���۲����ݣ�����NH3��CO2ͨ���������ͨ���۲����ݣ�����NH3��CO2�ķ�Ӧ���ʣ����������ñ�ˮ��ȴ��ԭ���ǽ����¶ȣ���߷�Ӧ��ת���ʣ����¶ȣ���ֹ��Ӧ������ɲ���ֽ⣩��
�ڴӷ�Ӧ��Ļ�����з������Ʒ��ʵ�鷽���ǹ��ˣ���д�������ƣ���Ϊ�˵õ������Ʒ��Ӧ��ȡ�ķ�����b����дѡ����ţ���
a����ѹ���Ⱥ�� b����ѹ40�����º�� c����ѹ���Ⱥ��
��3���Ƶõİ�������刺��ܺ���̼����李�̼����е�һ�ֻ����֣�
����Ʒ��������гɷ�̽��������д���пո�
��ѡ�Լ�������ˮ��ϡHNO3��BaCl2��Һ��Ba��OH��2��Һ��AgNO3��Һ��ϡ���ᣮ
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����������Ʒ���Թ��У���������ˮ�������ܽ⣮ | �õ���ɫ��Һ |
| ����2�����Թ��м��������BaCl2��Һ�����ã� | ��Һ����ǣ���֤�������к��У�NH4��2CO3�� |
| ����3��ȡ����2���ϲ���Һ���Թ��м���������Ba��OH��2��Һ�� | ��Һ������ǣ���֤�������в�����NH4HCO3�� |
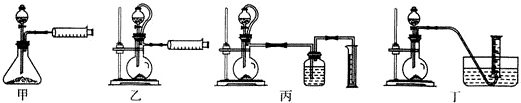
 ijʵ��С������ͼװ���Ʊ���������Һ����̽�������ʣ�
ijʵ��С������ͼװ���Ʊ���������Һ����̽�������ʣ���Ӧֹͣ��ȡϴ��ƿ����ɫ��Һ5mL�ֱ����������ʵ�飺
| ���� | ���� |
| a������ҺpH���������еμ�2�η�̪ | pH=13����Һ��죬5min����ɫ |
| b����������μ������� | ��Һ��ɻ���ɫ |
��2���������ϣ���̪�ı�ɫ��ΧΪ8.2��10���ҷ�̪��ǿ������Һ�к�ɫ����ȥΪ̽������a����Һ��ɫ��ԭ���ֲ���������ʵ�飺
| ���� | ���� |
| ȡ5mLpH=13NaOH��Һ�������еμ�2�η�̪ | ��Һ��죬30min����ɫ |
��3����С���ɲ���b��ý��ۣ�������Һ���Ե���ǿ��������Һ���ȶ����½���
�ٲ���b����Һ��ɻ���ɫ��ԭ��2H++ClO-+Cl-�TCl2��+H2O�������ӷ���ʽ��ʾ����
����ͬѧ��Ϊ�ɲ���b����������۲����Ͻ�����Ҫ��һ��ȷ�ϴ˽��۵�ʵ�鷽����ȡϴ��ƿ����Һ5mL����������μ������ᣬ�۲���Һ�Ƿ���Ϊ����ɫ��
��4����Ч�ȵĺ����Ǽ�⺬������������Ч������Ҫָ�꣮�����á���λ�����ĺ�������Һ�����������������ͷų����������������б�����һ���������Һ��Ч�Ⱥ�����5%���ϣ�С��ͬѧ��������ʵ��ⶨ��Ч�ȣ�
ȡ������Һ5g������20mL 0.5mol•L-1KI��Һ��10mL 2mol•L-1��������Һ���Ӽ��ε�����Һ����0.1mol•L-1Na2S2O3��Һ�ζ����ɵ�I2����ζ��յ�ʱ����Na2S2O320mL������֪��2S2O32-+I2�TS4O62-+2I-����ע�����ԭ������Cl35.5��Na23��S32��O 16��I 127��K39
�ٴﵽ�ζ��յ�ʱ��ʵ����������Һ��ɫ��ȥ�Ұ���Ӳ��ָ���
�ڴ�����Һ��Ч�Ⱥ���Ϊ1.42%������һλ��Ч���֣���
��ý��ۣ���ʵ���Ƶõ�����Һ�����ϣ�����ϡ������ϡ�������Ҫ��
 D��0.3 mol/��L��min��
D��0.3 mol/��L��min��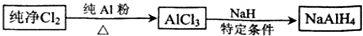
 ��
�� ����������������
����������������