��Ŀ����
4��Cl2��SO2����ʹƷ����Һ��ɫ��������ΪƷ����ӽṹ�е���ɫ�������������ṹ�����ı䣬���ɲ��ȶ�����ɫ�������Ư��ԭ����������ķ�Ӧ����ʽ��ʾ��
����˵����ȷ���ǣ�������
| A�� | ��Ʒ����Һ��ͬʱͨ��Cl2��SO2��Ư��Ч������� | |
| B�� | ���ȿ��ж�Ʒ����ɫ��ͨ��SO2����ͨ��Cl2����� | |
| C�� | ����ɫ����������У�19��̼ԭ�Ӷ����ܴ���ͬһƽ���� | |
| D�� | Ʒ����ӽṹ�У�����ԭ�Ӳ����ܴ���ͬһƽ���� |
���� A����Ʒ����Һ��ͬʱͨ��Cl2��SO2�������Ͷ�������Ӧ������������ᣬ������Ư���ԣ�
B�����������Ư�ײ��ȶ��������ֽ⣻
C����ɫ�����ﺬ�б���̼ԭ�ӣ����м���Ľṹ�ص㣻
D��Ʒ���к��а�����ԭ�Ӳ�������ͬһƽ���ϣ�
��� �⣺A����Ʒ����Һ��ͬʱͨ��Cl2��SO2�������Ͷ�������Ӧ������������ᣬ������Ư���ԣ�������Ʒ����ɫ����A����
B�����������Ư�ײ��ȶ��������ֽ⣬����ʱ�ָ�����ɫ��Ϊ��������B��ȷ��
C����ɫ�����ﺬ�б���̼ԭ�ӣ����м���Ľṹ�ص㣬19��̼ԭ�Ӳ����ܴ���ͬһƽ���ϣ���C����
D��Ʒ���к��а��������а����Ľṹ��ԭ�Ӳ�������ͬһƽ���ϣ���D��ȷ��
��ѡBD��
���� ���⿼���л���Ľṹ�������Լ������Ͷ��������Ư���ԣ�������ѧ���ķ�����������ѧ�����Ŀ��飬Ϊ��Ƶ���㣬ע����������Ϣ�����������Ԫ�ػ�����֪ʶ��ѧϰ���Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
8�����б仯�Ƿ��ȷ�Ӧ���ǣ�������
��̼��ȼ�� �ڽ��������ȱ�Ϊ��ɫ��ĩ ��Ũ����ϡ��
������طֽ������� ����ʯ����ˮ��Ӧ������ʯ�ң�
��̼��ȼ�� �ڽ��������ȱ�Ϊ��ɫ��ĩ ��Ũ����ϡ��
������طֽ������� ����ʯ����ˮ��Ӧ������ʯ�ң�
| A�� | �٢ۢ� | B�� | �ڢ� | C�� | �٢� | D�� | �ڢ� |
9�� ��֪ϡ������ǿ�������ᣬ��ԭ����һ��ΪNO����ͼ��Բ�ཻ����A��B��C��D�ֱ��ʾ�����ʼ�ķ�Ӧ�����и���Ӧ��Ӧ�����ӷ���ʽ��д����ȷ���ǣ�������
��֪ϡ������ǿ�������ᣬ��ԭ����һ��ΪNO����ͼ��Բ�ཻ����A��B��C��D�ֱ��ʾ�����ʼ�ķ�Ӧ�����и���Ӧ��Ӧ�����ӷ���ʽ��д����ȷ���ǣ�������
 ��֪ϡ������ǿ�������ᣬ��ԭ����һ��ΪNO����ͼ��Բ�ཻ����A��B��C��D�ֱ��ʾ�����ʼ�ķ�Ӧ�����и���Ӧ��Ӧ�����ӷ���ʽ��д����ȷ���ǣ�������
��֪ϡ������ǿ�������ᣬ��ԭ����һ��ΪNO����ͼ��Բ�ཻ����A��B��C��D�ֱ��ʾ�����ʼ�ķ�Ӧ�����и���Ӧ��Ӧ�����ӷ���ʽ��д����ȷ���ǣ�������| A�� | Fe+4H++NO3-�TFe3++NO��+2H2O | |
| B�� | Fe3O4+8H+�TFe2++2Fe3++4H2O | |
| C�� | Fe��OH��3+3H+�TFe3++3H2O | |
| D�� | 3Fe��OH��2+10H++NO3-�T3Fe3++NO��+8H2O |
12�����и�������һ���ܴ���������ǣ�������
| A�� | ����ɫ��Һ�У�NH4+��Fe2+��SO42-��CO32- | |
| B�� | �ں�����Ba2+��Һ�У�NH4+��Na+��Cl-��OH- | |
| C�� | ��ǿ����Һ�У�Na+��K+��Cl-��SO32- | |
| D�� | �����Ե���Һ�У�K+��Fe2+��Cl-��CH3COO- |
19������������ȷ���ǣ�������
��ͬλ�أ�1H��2H2��3H ��ͬ�������壺C60�����ʯ��ʯī �����������CO2��NO��SO3 �ܻ���ˮ������ˮ����ˮú�� �ݵ���ʣ������������ᡢʯ�� �ɱ���Һ�ȡ��Ҵ����Ƿǵ���ʣ�
��ͬλ�أ�1H��2H2��3H ��ͬ�������壺C60�����ʯ��ʯī �����������CO2��NO��SO3 �ܻ���ˮ������ˮ����ˮú�� �ݵ���ʣ������������ᡢʯ�� �ɱ���Һ�ȡ��Ҵ����Ƿǵ���ʣ�
| A�� | �ڢ� | B�� | �ڢݢ� | C�� | �ڢܢݢ� | D�� | �٢ڢۢܢݢ� |
9��ͬ��ͬѹ�£������ʵ�����NO��NO2������ͬ�ģ�������
| A�� | ��ԭ���� | B�� | ���� | C�� | ԭ���� | D�� | ��� |
16��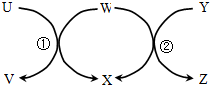 ��ͼ��U��Z�����������ʵ���������ʵ��ͼʾ��ͷ����һ��ת�����ҷ�Ӧ�١��ھ�Ϊ�û���Ӧ�������������������ǣ�������
��ͼ��U��Z�����������ʵ���������ʵ��ͼʾ��ͷ����һ��ת�����ҷ�Ӧ�١��ھ�Ϊ�û���Ӧ�������������������ǣ�������
 ��ͼ��U��Z�����������ʵ���������ʵ��ͼʾ��ͷ����һ��ת�����ҷ�Ӧ�١��ھ�Ϊ�û���Ӧ�������������������ǣ�������
��ͼ��U��Z�����������ʵ���������ʵ��ͼʾ��ͷ����һ��ת�����ҷ�Ӧ�١��ھ�Ϊ�û���Ӧ�������������������ǣ�������| ��� | U | W | Y | X |
| �� | Na | H2O | Na2O2 | NaOH |
| �� | Fe | H2O | C | H2 |
| �� | HBr | Cl2 | CH4 | HCl |
| �� | CuCl2��aq�� | Al | HCl��aq�� | AlCl3��aq�� |
| A�� | �ڢ� | B�� | �ڢ� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |
13���������ŷḻ�ĺ�ˮ��Դ������������Դ�ͱ�����������ʡ�ɳ�����չ����Ҫ��֤��
��1����ˮ������������Եõ���ˮ�Ȼ�þ����ˮ�Ȼ�þ�ǹ�ҵ��ȡþ��ԭ�ϣ���д������ˮ�Ȼ�þ��ȡ����þ�Ļ�ѧ��Ӧ����ʽMgCl2 $\frac{\underline{\;���\;}}{\;}$Mg+Cl2����
��2��ij���������������л��������Cu2+��Pb2+����ˮ���ŷ�ǰ���ó�������ȥ���������ӣ������������ݣ�����ΪͶ��Na2S��ѡ�Na2S����NaOH����Ч�����ã�
��3�����������ڽ��յ���Դ������ռ�ϴ���أ������ŷų���SO2�����һϵ�л�������̬���⣮���ú�ˮ������һ����Ч�ķ������乤��������ͼ��ʾ��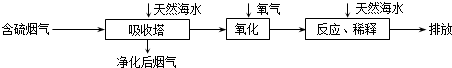
����Ȼ��ˮ��pH��8��ˮ����Ҫ����Na+��K+��Ca2+��Mg2+��Cl-��SO42-��Br-��CO32-��HCO3-�����ӣ��������ӷ���ʽ������Ȼ��ˮ�������Ե�ԭ��CO32-+H2O?HCO3-+OH-�� HCO3-+H2O?H2CO3+OH-����дһ������
��ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ����������Ȼ��ˮ���պ���������ģ��ʵ�飬ʵ������ͼ��ʾ��

�������ͼʾʵ��������������һ��Ũ�Ⱥ���������SO2������Ч�ʣ����һ�����������飺���ͺ��������¶ȣ������٣���
����Ȼ��ˮ�����˺��������������H2SO3��HSO3-�ȷ��ӻ����ӣ�ͨ�����������Ļ�ѧԭ����2H2SO3+O2=4H++2SO42-��2HSO3-+O2=2H++2SO42-����дһ����ѧ����ʽ�����ӷ���ʽ����������ġ���ˮ����Ҫ�����������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ�����к͡�ϡ�;�����������ˮ�����ɵ��ᣨH+����
��1����ˮ������������Եõ���ˮ�Ȼ�þ����ˮ�Ȼ�þ�ǹ�ҵ��ȡþ��ԭ�ϣ���д������ˮ�Ȼ�þ��ȡ����þ�Ļ�ѧ��Ӧ����ʽMgCl2 $\frac{\underline{\;���\;}}{\;}$Mg+Cl2����
��2��ij���������������л��������Cu2+��Pb2+����ˮ���ŷ�ǰ���ó�������ȥ���������ӣ������������ݣ�����ΪͶ��Na2S��ѡ�Na2S����NaOH����Ч�����ã�
| ���ܵ���� | Cu��OH��2 | CuS | Pb��OH��2 | PbS |
| Ksp | 4.8��10-20 | 6.3��10-36 | 1.2��10-15 | 1.0��10-28 |

����Ȼ��ˮ��pH��8��ˮ����Ҫ����Na+��K+��Ca2+��Mg2+��Cl-��SO42-��Br-��CO32-��HCO3-�����ӣ��������ӷ���ʽ������Ȼ��ˮ�������Ե�ԭ��CO32-+H2O?HCO3-+OH-�� HCO3-+H2O?H2CO3+OH-����дһ������
��ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ����������Ȼ��ˮ���պ���������ģ��ʵ�飬ʵ������ͼ��ʾ��

�������ͼʾʵ��������������һ��Ũ�Ⱥ���������SO2������Ч�ʣ����һ�����������飺���ͺ��������¶ȣ������٣���
����Ȼ��ˮ�����˺��������������H2SO3��HSO3-�ȷ��ӻ����ӣ�ͨ�����������Ļ�ѧԭ����2H2SO3+O2=4H++2SO42-��2HSO3-+O2=2H++2SO42-����дһ����ѧ����ʽ�����ӷ���ʽ����������ġ���ˮ����Ҫ�����������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ�����к͡�ϡ�;�����������ˮ�����ɵ��ᣨH+����
14�������йػ�ѧ�����ʾ��ȷ���ǣ�������
| A�� | HCl�ĵ���ʽ�� | B�� | ${\;}_{8}^{18}$O2-���ӵ�ԭ�ӽṹʾ��ͼ�� | ||
| C�� | CO2���ӵĽṹʽ��O=C=O | D�� | ���Ȼ�̼���ӱ���ģ�ͣ� |