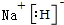��Ŀ����
18��FeF3�Ǽص�ص������������ʣ���ͨ�����з����Ʊ���
��1��������й�������������ǽ��������������������ӣ�д��H2O2��FeCl2��NaOH�����·�Ӧ�����ӷ���ʽ��H2O2+2Fe 2++4OH-=2Fe��OH��3��
��2�����˺�����Һ�Ի��ǣ����ܵ�ԭ���ǹ���ʱ��Һ������ֽ��Ե����ֽ����ʵ�鲽���������������ϩ������ʢ�ų�������������ͨ�մ�������ԭ���������������ͨ�մ��еĶ������跴Ӧ
��3������ڵõ��������У���ˮ��������Ҫ����HF
��4����������Ƶ�97.0gFeF3•xH2O������������۵õ�59.5g3FeF3•H2O����x=$\frac{50}{9}$��
���� �������̿�֪���Ȼ�����Һ�к����Ȼ��������ʣ�����˫��ˮ���Խ��������������������ӣ��ټ��������������������������������������м�����ᣬ�������ɵ�FeF3•xH2O�������ٸ���ɵ�FeF3•H2O��
��1��˫��ˮ�ܽ��������������������ӣ����������Ƶ���������������������
��2������ʱ��Һ������ֽ��Ե����ֽ����ʹ��Һ���ǣ���Ϊ���������������跴Ӧ�����Բ�������ͨ�մ������ݴ˴��⣻
��3��HF�ӷ����ݴ��жϣ�
��4�����ݷ�ӦFeF3•xH2O=FeF3•H2O+��x-1��H2O�����FeF3•xH2O��FeF3•xH2O�������ɼ����x��
��� �⣺�������̿�֪���Ȼ�����Һ�к����Ȼ��������ʣ�����˫��ˮ���Խ��������������������ӣ��ټ��������������������������������������м�����ᣬ�������ɵ�FeF3•xH2O�������ٸ���ɵ�FeF3•H2O��
��1��˫��ˮ�ܽ��������������������ӣ����������Ƶ�����������������������Ӧ�����ӷ���ʽΪH2O2+2Fe 2++4OH-=2Fe��OH��3����
�ʴ�Ϊ�����������������������ӣ�H2O2+2Fe 2++4OH-=2Fe��OH��3����
��2������ʱ��Һ������ֽ��Ե����ֽ����ʹ��Һ���ǣ���Ϊ�����������ͨ�մ��еĶ������跴Ӧ�����Բ�������ͨ�մ�������
�ʴ�Ϊ������ʱ��Һ������ֽ��Ե����ֽ���������������ͨ�մ��еĶ������跴Ӧ��
��3��HF�ӷ������Բ���ڵõ��������У���ˮ��������Ҫ����HF��
�ʴ�Ϊ��HF��
��4�����ݷ�ӦFeF3•xH2O=FeF3•H2O+��x-1��H2O��
113+18x 131
97.0g 59.5g
������$\frac{113+18x}{97.0}$=$\frac{131}{59.5}$��x=$\frac{50}{9}$��
�ʴ�Ϊ��$\frac{50}{9}$��
���� ���⿼���������Ʊ����漰���ӷ���ʽ����д��ʵ�������������ѧ����ȣ��е��Ѷȣ�ע����㷽���ͻ�������������Ӧ�ã����ջ����ǹؼ������ػ���֪ʶ�ͻ��������Ŀ��飮

| A�� |  ʯ�͵ķ��� | B�� |  ������������ˮ | ||
| C�� | 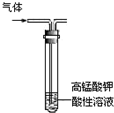 ��ȥ�����е���ϩ | D�� | 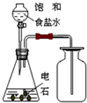 ��ȡ���ռ���Ȳ���� |
| A�� | ͬ���칹�壺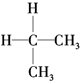 �� ��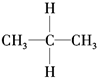 | B�� | ������CH4�� ��CH2�TCH2 ��CH2�TCH2 | ||
| C�� | ���ࣺ�����ǡ����ǡ���ά�� | D�� | �л��߷��ӣ����ϡ�����֬ |
| A�� | K+��Ca2+��Cl-��SO42- | B�� | NH4+��HCO3-��Cl-��K+ | ||
| C�� | Cl-��Na+��NO3-��Ca2+ | D�� | MnO4-��NO3-��Na+��Cl- |
������ͭ˿����Ũ���ᣬ���ȣ�
������������ɫ����������ʱ�����ͭ˿��ֹͣ���ȣ�
����ȴ�ӷ�Ӧ��Ļ�����з������ɫ������ϴ�������ﱸ�ã�
�ش��������⣺
��1��������������Ļ�ѧʽΪSO2��
��2������ Cu2+��Һ�еμ�K4[Fe��CN��6]��Һ���ܲ������ɫ�������ֽ�������ɫ��������ϡ�����У�������Ժ��ٵμ�K4[Fe��CN��6]��Һ��δ�����ɫ�������ɴ����ý����Ǻ�ɫ�����в�����CuO��
��3��Ϊ֤����ɫ��������ͭ�������������ʵ�飺
| װ�� | ���� | ���ۼ����� |
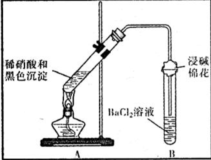 | ��A�Թ��к�ɫ�������ܽ� ��A�Թ��Ϸ����ֺ���ɫ���� ��B�Թ��г��ְ�ɫ���� | a�������˵����ɫ�������� ��ԭ���ԣ� b���Թ�B�в�����ɫ�������ܷ�Ӧ�����ӷ���ʽΪ NO2+SO2+Ba2++H2O�TBaSO4��+NO��+2H+ |
��5��Ϊ�ⶨ��ɫ������Cu2S �İٷֺ�����ȡ0.2g ��������ú�ɫ��������������Һ���� 40.0mL 0.075mol/L KMnO4��Һ������������Ӧ���£�
8MnO4-+5Cu2S+44H+�T10Cu2++5SO2��+8Mn2++22H2O
6MnO4-+5CuS+28H+�T5Cu2++5SO2��+6Mn2++14H2O
��Ӧ�������Һ���Ͼ�SO2�������ĸ��������Һǡ����35.0mL 0.1mol/L ��NH4��2Fe��SO4��2 ��Һ��Ӧ��ȫ����������Cu2S ����������Ϊ40%��
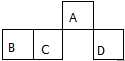 ������Ԫ��A��B��C��D��Ԫ�����ڱ��е����λ����ͼ������Aԭ�ӵ����������������ڲ��������3���������ж���ȷ���ǣ�������
������Ԫ��A��B��C��D��Ԫ�����ڱ��е����λ����ͼ������Aԭ�ӵ����������������ڲ��������3���������ж���ȷ���ǣ�������| A�� | ԭ�Ӱ뾶��rD��rC��rB��rA | |
| B�� | ��DԪ�ص�����Һһ�������� | |
| C�� | �⻯������ȶ��ԣ�C��D | |
| D�� | D�ĵ�������A�γɵ��⻯�ﷴӦ���ɾ���Ư���Ե����� |
| A | B | |
| C | D |
��1��Aλ��Ԫ�����ڱ������ڣ�VA�壬���⻯��ķ���ʽ��NH3��
��2�����������У���ȷ����a������ĸ����
a���ȶ��ԣ�A���⻯�C���⻯�� b����ԭ�ԣ�B2-��D2-
c�����ԣ�H4CO4��H2DO4 d������ϼ�ֵ��D=B��A��C
��3��DB2ͨ�����й������̿��ƻ���ҵԭ��H2DB4�������ԴH2��

��ԭ�����DB2���뷴Ӧ�ĵ缫Ϊ������д���缫��ӦʽSO2+2H2O-2e-=4H++SO42-������5mol DB2�μӷ�Ӧ��������Ӧ������ģ��������ɱ�״����112L H2��
��Ϊ����������ķ���Ч����ȡ������H2DB4��Һ���Թܣ���������μ���AgNO3��Һ����ַ�Ӧ�����۲쵽����ɫ�����������������ɰ�ɫ������֤������Ч���Ϻã�
�۽��ù����������ܷ�Ӧ�Ļ�ѧ����ʽ��ʾΪ��SO2+2H2O=H2SO4+H2��
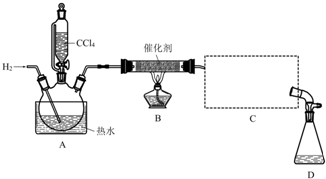
| ���� | ��Է������� | �ܶ�/��g•mL-1�� | �е�/�� | ˮ���ܽ��� |
| CHCl3 | 119.5 | 1.50 | 61.3 | ���� |
| CCl4 | 154 | 1.59 | 76.7 | ���� |
�ټ���װ�������ԣ��ڿ�ʼͨ��H2�� �۵�ȼB���ƾ��ƣ�
����A��ˮ���м�����ˮ����ͨC������װ�õ���ˮ��
��������ƿ�е���20mLCCl4��
��Ӧ������ֹͣ���ȣ���D����ƿ���ռ�����Һ��ֱ�������NaHCO3��Һ��ˮϴ�ӣ��ֳ��IJ������������ˮCaCl2���壬���ú���ˣ�
�߶���Һ�����������õ��ȷ�15g����ش�
��1��������ںͲ���۵�˳��ߵ�����ʵ���в����IJ����������Ϊ����ʱ����������������ը�����ɵ��ȷ±�����������
��2��B���з�����Ҫ��Ӧ�Ļ�ѧ����ʽΪCCl4+H2$��_{��}^{����}$CHCl3+HCl��
��3��C����Ӧѡ�õ�������ΪB����ѡ����ĸ������ˮӦ�Ӹ������ܵ�a���a����b�����ڽ��룮
��4��������У���ˮϴ�ӵ�Ŀ��Ϊϴ��NaHCO3��NaCl��
��5����ʵ���У��ȷµIJ���Ϊ61%��
��6���ȷ��ڿ������ܱ�������������HCl������COCl2�����÷�Ӧ�Ļ�ѧ����ʽΪ2CHCl3+O2=2COCl2+2HCl��
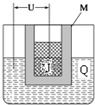 ��֪X��Y��Z��J��Q���ֶ���������Ԫ�أ�ԭ��������������Ԫ��Z�ڵؿ��к�����ߣ�JԪ�ص���ɫ��Ӧ�ʻ�ɫ��Q�������������������������Ϊ3��8��X����J�γ����ӻ������J+�İ뾶����X-�İ뾶��Y2�ǿ�����Ҫ�ɷ�֮һ����ش�
��֪X��Y��Z��J��Q���ֶ���������Ԫ�أ�ԭ��������������Ԫ��Z�ڵؿ��к�����ߣ�JԪ�ص���ɫ��Ӧ�ʻ�ɫ��Q�������������������������Ϊ3��8��X����J�γ����ӻ������J+�İ뾶����X-�İ뾶��Y2�ǿ�����Ҫ�ɷ�֮һ����ش�