��Ŀ����
6�����к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȣ������²��裺��1�����ƴ���Һ����5.0g�����������ʣ����ʲ������ᷴӦ���Ĺ����ռ���Ʒ����1000mL��Һ�����ձ��Ͳ����⣬����Ҫ�IJ���������1000mL����ƿ
��2���ζ�����ʢװ0.1000mol/L�������ҺӦ��ʹ����ʽ�ζ��ܣ�
�ڵζ�ʱ˫��Ӧע��۲���ƿ����Һ��ɫ�ı仯��
��3���й����ݼ�¼���£�
| �ⶨ��� | ������Һ�������mL�� | ���������Һ�������mL�� | |
| �ζ�ǰ���� | �ζ������ | ||
| 1 | 20.00 | 0.50 | 20.78 |
| 2 | 20.00 | 1.20 | 21.32 |
��4��������ۣ���ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족��
��������ˮ��ϴ��ƿ����ʹ�ⶨ�����Ӱ�죻
���ڵζ������в�����������Һ������ƿ�⣬��ʹ�ⶨ���ƫ�ߣ�
�۶���ʱ���ζ�ǰ���ӣ��ζ����ӣ���ʹ�ⶨ���ƫ�ͣ�
��װ��Һ֮ǰ��û���ñ�Һ��ϴ�ζ��ܣ���ʹ�ⶨ���ƫ�ߣ�
���� ��1������һ�����ʵ���Ũ�ȵ�����������Һ��Ҫ����������ƿ����ͷ�ιܡ��ձ�������������ƽ��ҩ�ȣ�
��2����������ҺӦ����ʽ�ζ���ʢ�ţ��ڵζ�ʱ�۾�ע����ƿ����ɫ�仯��
��3��������������������ƽ�������Ȼ������������Ƶ����ʵ������ټ����ռ���Ʒ�Ĵ��ȣ�
��4����������ˮ��ϴ��ƿ����Ӱ�������������ʵ�����
�ڵζ������в����������ƿ�⣬������������ƫ�ࣻ
�۵ζ�ǰ���ӡ��ζ����ӣ������������ƫС��
��δ�ñ�Һ��ϴ�ζ��ܣ������������ƫ��
��� �⣺��1������1000mLһ�����ʵ���Ũ�ȵ�����������Һ��Ҫ������1000mL����ƿ����ͷ�ιܡ��ձ�������������ƽ��ҩ�ף��������ƴ���Һ����Ҫ�IJ���������1000mL����ƿ������������ͷ�ιܣ��ձ���
�ʴ�Ϊ��1000mL����ƿ��
��2����ʢװ0.1000mol•L-1�����ҺӦ��ʹ����ʽ�ζ��ܣ��ڵζ�ʱ˫��Ӧע��۲���ƿ����Һ��ɫ�ı仯��
�ʴ�Ϊ���
�ڵζ�ʱ˫��Ӧע��۲���ƿ����Һ��ɫ�ı仯���ʴ�Ϊ��ע��۲���ƿ����Һ��ɫ�ı仯��
��3������������������ֱ�Ϊ��20.28ml��20.12ml���������������ƽ�����Ϊ20.20mL��20.00mL����������Һ��n��NaOH��=n��HCl��=0.10mol/L��0.0202L=0.00202mol������1000mL������Һ����m���ռ�T0.00202mol��50��40g/mol=4.02g�������ռ�Ĵ��Ȧأ��ռ=$\frac{4.02g}{5.0g}$��100%=80.4%��
�ʴ�Ϊ��80.4%��
��4���ٳ�ϴ��ƿ����Ӱ�������������ʵ���������Ӱ��ⶨ������ʴ�Ϊ����Ӱ�죻
�ڵζ������в����������ƿ�⣬������������ƫ�࣬�ⶨ���ƫ�ʴ�Ϊ��ƫ�ߣ�
�۵ζ�ǰ���ӡ��ζ����ӣ������������ƫС���ⶨ���ƫС���ʴ�Ϊ��ƫ�ͣ�
��װ��Һ֮ǰ��û���ñ�Һ��ϴ�ζ��ܣ���ҺŨ�ȼ�С�����ĵ��������࣬�ⶨ���ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
���� ���⿼���˵ζ�����������ʱҪ�淶���������ʱҪ���Ƿ�Ӱ���������������������ƫ���ƫ�ߣ��������ƫС������ƫС������Ӱ��������������Ӱ�죮

�����DZ궨KMnO4��Һ��ʵ�鲽�裺
����һ��������Ũ��ԼΪ0.10mol•L-1�ĸ��������Һ500mL��
�������ȡ0.02mol•L-1 ��Na2C2O420.00mL������ƿ�У�����ϡ�����ữ���ò���һ������������Һ���еζ�������ƽ��ʵ������ݼ�¼�ڱ��У�
| ƽ��ʵ���� | Na2C2O4��Һ ��mL�� | �ζ�����ʼ������mL�� | �ζ��ܵζ��յ������mL�� |
| 1 | 20.00 | 0.00 | 21.18 |
| 2 | 20.00 | 1.02 | 21.00 |
| 3 | 20.00 | 1.18 | 21.20 |
��1������һ��Ҫ�õ�����Ҫ���������dz��ձ�����������裮
��2��������еζ�����ѡ�õζ��ܣ����ʽ����ʽ�����ζ��յ���жϷ�����
��3�������ζ������ᵼ�²������ƫ�ߵ���
A���ζ��ܵζ�ǰ�����ݣ��ζ���������ʧ B����ƿϴ�Ӻ�û�и���
C���ζ��յ�ʱ������ʱ���� D���ζ�ʱ��ƿ����Һ�彦��
E��һ�θ��������Һ������ƿ����δ����
��4����ʵ���������ݼ��㣬KMnO4��Һ��Ũ��Ϊmol•L-1��
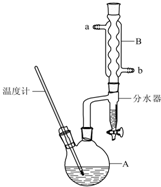 �����ѳ������л���Ӧ���ܼ���ʵ�����Ʊ������ѵķ�Ӧ����Ҫʵ��װ�����£�
�����ѳ������л���Ӧ���ܼ���ʵ�����Ʊ������ѵķ�Ӧ����Ҫʵ��װ�����£�2CH3CH2CH2CH2OH$?_{135��}^{Ũ����}$��CH3CH2CH2CH2��O+H2O ��Ӧ��������������������£�
| ��Է������� | �е�/�� | �ܶ�/g•cm3 | ˮ���ܽ��� | |
| ������ | 74 | 117.2 | 0.8109 | �� |
| ������ | 130 | 142.0 | 0.7704 | �������� |
�ٽ�6mLŨ�����37g����������һ��˳�����ӵ�A�У����Ӽ�����ʯ��
�ڼ���A�з�ӦҺ��Ѹ��������135�棬ά�ַ�Ӧһ��ʱ�䣮
�����ᴿ��
�۴�A��Һ����ȴ���仺������ʢ��70mLˮ�ķ�Һ©���У���ҡ���ã���Һ�ôֲ��
�ֲܴ���������40mLˮ��20mLNaOH��Һ��40mLˮϴ�ӣ���Һ�����Լ3g��ˮ�Ȼ��ƿ���������һ��ʱ�����ȥ�Ȼ��ƣ�
�ݽ������������Ĵֲ�����������ռ���֣��ô���������13g��
�ش��������⣺
��1���������Ũ�����������������˳��Ϊ�ȼ������������ټ���Ũ���ᣮ
��2������Aǰ�����ȴ�b���a����b��������B��ͨ��ˮ��
��3����Ӧ�����л�۲쵽��ˮ�����ռ���Һ�����ʣ��ҷ�Ϊ�������㣬���ŷ�Ӧ�Ľ��У���ˮ����Һ��������������ʱ���ϲ�Һ�������֧���Զ�����A����ˮ�����ϲ�Һ�����Ҫ�ɷ�Ϊ�����������÷�ˮ�����˿�������������������ʣ����������Ϸ����ˮ����ʹƽ�������ƶ����ã������йػ�ѧ���ۻش𣩣�
��4������۵�Ŀ���dz���ϴȥŨ���ᣬ��ҡ���ã��ֲ���Ӧ�ڷ�Һ©�����ϣ���ϡ����¡����ڷ������
��5�����¶ȹ��ᷢ������Ӧ����ϩ�������ܵķ�Ӧ����ʽΪCH3CH2CH2CH2OH$��_{��}^{Ũ����}$ CH3CH2CH=CH2��+H2O��
��6��������У���������ʱӦ�ռ�142�����ҵ���֣���ʵ���У������ѵIJ���Ϊ40.0%��
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����ʳ�����������/mL | 0.02 | 0.03 | 0.00 |
| ����ʳ������ն���/mL | 25.01 | 25.04 | 25.02 |
| �������Ʊ�Һ�����������/mL | 0.01 | 0.03 | 0.04 |
| �������Ʊ�Һ������ն���/mL | 12.52 | 12.55 | 12.58 |
| A�� | �ܽ⡢���ˡ����� | B�� | �ܽ⡢���ˡ�ϴ�ӡ����� | ||
| C�� | �ܽ⡢���ˡ��ᾧ | D�� | �ܽ⡢�ᾧ������ |
 2SO2��g��+O2��g��?2SO3��g����H=-198kJ•mol-1��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
2SO2��g��+O2��g��?2SO3��g����H=-198kJ•mol-1��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺ ��ʵ��������֪Ũ�ȵ�����ζ�ijδ֪Ũ�ȵ�NaOH��Һ��װ�úͲ�������ͼ��ʾ����ش�
��ʵ��������֪Ũ�ȵ�����ζ�ijδ֪Ũ�ȵ�NaOH��Һ��װ�úͲ�������ͼ��ʾ����ش�