��Ŀ����
��1��д���������ʵĵ��뷽��ʽ
A��NaHCO3 B��NaHSO4
��2��д���������ʵ�ˮ������ӷ���ʽ
A��CH3COONa B��Fe2��SO4��3
��3��д���������ܵ���ʵ��ܽ�ƽ�����ʽ��
A��Al��OH��3 B��AgCl
��4��д���������ʷ�Ӧ���Ȼ�ѧ����ʽ
A��0.4mol��N2H4��Һ̬����������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ���������ų�256.65kJ������ ��
B��������ȼ������285.8kJ/mol�� ��
A��NaHCO3
��2��д���������ʵ�ˮ������ӷ���ʽ
A��CH3COONa
��3��д���������ܵ���ʵ��ܽ�ƽ�����ʽ��
A��Al��OH��3
��4��д���������ʷ�Ӧ���Ȼ�ѧ����ʽ
A��0.4mol��N2H4��Һ̬����������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ���������ų�256.65kJ������
B��������ȼ������285.8kJ/mol��
���㣺���뷽��ʽ����д,�Ȼ�ѧ����ʽ
ר�⣺��ѧ��Ӧ�е������仯,����ƽ������Һ��pHר��
��������1��A��̼������Ϊǿ����ʣ���Һ����ȫ����������Ӻ�̼��������ӣ�
B��������������Һ�е���������ӡ������Ӻ���������ӣ�
��2��A����������ӽ��ˮ����������ӣ���Һ��ʾ���ԣ�
B����������Һ�У������ӽ��ˮ��������������ӣ���Һ��ʾ���ԣ�
��3��������������ܽ�ƽ�ⷽ��ʽ����дԭ����н��
��4��A����Ӧ����ʽΪ��N2H4+2H2O2=N2+4H2O������0.4molҺ̬�·ų�������������1molҺ̬�·ų�������������д���Ȼ�ѧ����ʽ��
B������������ȼ���ȼ��Ȼ�ѧ����ʽ����дԭ����н��
B��������������Һ�е���������ӡ������Ӻ���������ӣ�
��2��A����������ӽ��ˮ����������ӣ���Һ��ʾ���ԣ�
B����������Һ�У������ӽ��ˮ��������������ӣ���Һ��ʾ���ԣ�
��3��������������ܽ�ƽ�ⷽ��ʽ����дԭ����н��
��4��A����Ӧ����ʽΪ��N2H4+2H2O2=N2+4H2O������0.4molҺ̬�·ų�������������1molҺ̬�·ų�������������д���Ȼ�ѧ����ʽ��
B������������ȼ���ȼ��Ȼ�ѧ����ʽ����дԭ����н��
���
�⣺��1��A��̼��������ˮ�е���������Ӻ�̼��������ӣ����뷽��ʽΪ��NaHCO3�TNa++HCO3-��
�ʴ�Ϊ��NaHCO3�TNa++HCO3-��
B��NaHSO4��ǿ����ʣ�NaHSO4��ˮ�е���������Ӻ���������Ӻ������ӣ�NaHSO4�TNa++H++SO42-��
�ʴ�Ϊ��NaHSO4�TNa++H++SO42-��
��2��A����������Һ�У����������ˮ�⣬��Һ��ʾ���ԣ���ˮ�ⷽ��ʽΪ��CH3COO-+H2O?CH3COOH+OH-��
�ʴ�Ϊ��CH3COO-+H2O?CH3COOH+OH-��
B����������Һ��������ˮ���������������������ӣ������ӵ�ˮ�ⷽ��ʽΪ��Fe3++3H2O?Fe��OH��3+3H+��
�ʴ�Ϊ��Fe3++3H2O?Fe��OH��3+3H+��
��3��A��Al��OH��3�ܽ�ƽ�ⷽ��ʽΪ��Al��OH��3��s��?Al3+��aq��+3OH-��aq����
�ʴ�Ϊ��Al��OH��3��s��?Al3+��aq��+3OH-��aq����
B���Ȼ�������Һ�е��ܽ�ƽ�ⷽ��ʽΪ��AgCl��s��?Ag+��aq��+Cl-��aq����
�ʴ�Ϊ��AgCl��s��?Ag+��aq��+Cl-��aq����
��4��A����Ӧ����ʽΪ��N2H4+2H2O2�TN2+4H2O��0.4molҺ̬�·ų�256.65KJ����������1molҺ̬�·ų�������Ϊ��
=641.625kJ��
���Է�Ӧ���Ȼ�ѧ����ʽΪ��N2H4��g��+2H2O2��l���TN2��g��+4H2O��g����H=-641.625kJ/mol��
�ʴ�Ϊ��N2H4��g��+2H2O2��l��=N2��g��+4H2O��g����H=-641.625kJ/mol��
B������ȼ�����ȶ��IJ�����Һ̬ˮ������ȼ���Ƿ��ȷ�Ӧ��������ȼ�յ��Ȼ�ѧ����ʽΪ��2H2��g��+O2��g���T2H2O��l����H=-571.6 kJ/mol��
�ʴ�Ϊ��2H2��g��+O2��g���T2H2O��l����H=-571.6 kJ/mol��
�ʴ�Ϊ��NaHCO3�TNa++HCO3-��
B��NaHSO4��ǿ����ʣ�NaHSO4��ˮ�е���������Ӻ���������Ӻ������ӣ�NaHSO4�TNa++H++SO42-��
�ʴ�Ϊ��NaHSO4�TNa++H++SO42-��
��2��A����������Һ�У����������ˮ�⣬��Һ��ʾ���ԣ���ˮ�ⷽ��ʽΪ��CH3COO-+H2O?CH3COOH+OH-��
�ʴ�Ϊ��CH3COO-+H2O?CH3COOH+OH-��
B����������Һ��������ˮ���������������������ӣ������ӵ�ˮ�ⷽ��ʽΪ��Fe3++3H2O?Fe��OH��3+3H+��
�ʴ�Ϊ��Fe3++3H2O?Fe��OH��3+3H+��
��3��A��Al��OH��3�ܽ�ƽ�ⷽ��ʽΪ��Al��OH��3��s��?Al3+��aq��+3OH-��aq����
�ʴ�Ϊ��Al��OH��3��s��?Al3+��aq��+3OH-��aq����
B���Ȼ�������Һ�е��ܽ�ƽ�ⷽ��ʽΪ��AgCl��s��?Ag+��aq��+Cl-��aq����
�ʴ�Ϊ��AgCl��s��?Ag+��aq��+Cl-��aq����
��4��A����Ӧ����ʽΪ��N2H4+2H2O2�TN2+4H2O��0.4molҺ̬�·ų�256.65KJ����������1molҺ̬�·ų�������Ϊ��
| 256.65kJ |
| 0.4 |
���Է�Ӧ���Ȼ�ѧ����ʽΪ��N2H4��g��+2H2O2��l���TN2��g��+4H2O��g����H=-641.625kJ/mol��
�ʴ�Ϊ��N2H4��g��+2H2O2��l��=N2��g��+4H2O��g����H=-641.625kJ/mol��
B������ȼ�����ȶ��IJ�����Һ̬ˮ������ȼ���Ƿ��ȷ�Ӧ��������ȼ�յ��Ȼ�ѧ����ʽΪ��2H2��g��+O2��g���T2H2O��l����H=-571.6 kJ/mol��
�ʴ�Ϊ��2H2��g��+O2��g���T2H2O��l����H=-571.6 kJ/mol��
���������⿼���˵��뷽��ʽ��ˮ�ⷽ��ʽ���Ȼ�ѧ����ʽ��������ĵ��뷽��ʽ����д����Ŀ�Ѷ��еȣ����������ϴ��漰��֪ʶ��϶࣬��ֿ�����ѧ������ѧ֪ʶ�����������

��ϰ��ϵ�д�
�����Ŀ
��NA���������ӵ�����������˵����ȷ���ǣ�������
| A����״���£�22.4LCl2��HCl�Ļ������������������Ϊ2 NA |
| B����״���£�11.2L �Ҵ������ķ�����Ϊ0.5NA |
| C��0.1molCH4�����ĵ�����һ��ΪNA |
| D��22.4 L N2�������ķ�����һ��ΪNA |
������Һ�����Ӽ���Ľ�����ȷ���ǣ�������
| A����ϡ���������ɫ��ζ���壬������ͨ�����ʯ��ˮ��ʯ��ˮ����ǣ�ԭ��Һ���ܺ�CO32- |
| B������BaCl2��Һ�а�ɫ�����������ټ����ᣬ������ʧ��ԭ��Һһ������SO42- |
| C������AgNO3��Һ�а�ɫ����������ԭ��Һһ������Cl- |
| D������Na2CO3��Һ�а�ɫ�����������ټ����ᣬ��ɫ������ʧ��ԭ��Һ��һ������Ba2+ |
�ӷ���Ƕȣ�����˵����ȷ���ǣ�������
| A��ˮ������轺�������� |
| B��Ư��Һ��Ư�۾�����Ҫ�ɷ־�Ϊ���� |
| C��NO2��SO3���������������� |
| D�����ᡢһˮ�ϰ�������������� |
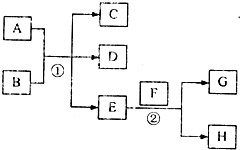 ��֪������EΪ��ɫ��ζ��Һ�壬FΪ����ɫ��ĩ��GΪ��������ɫ���壨��Ӧ��������ʡ�ԣ�
��֪������EΪ��ɫ��ζ��Һ�壬FΪ����ɫ��ĩ��GΪ��������ɫ���壨��Ӧ��������ʡ�ԣ�