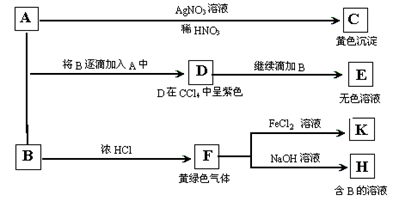
��Ŀ����
��11�֣�A��B��C��D��E��F�������������±仯��ϵ��E�ǵ���ɫ��ĩ���жϣ�
��1��д��A��B��C��D��E��F�Ļ�ѧʽ��
B.________��C.________��D.________��E��________��
��2��д���йط�Ӧ�Ļ�ѧ����ʽ(�����ӷ�Ӧ��ֱ��д���ӷ���ʽ)
E��B�� _______________��C��F�� _______________________��F��C�� ________________________��
��3������ʵ�鷽���У�����ȷ�ⶨNa2CO3��NaHCO3�������Na2CO3������������
A. ȡa�˻�����ּ��ȣ�����b��
B. ȡa�˻����������ϡ�����ַ�Ӧ�����ȡ����ɡ����գ���b�˹���
C. ȡa�˻����������ϡ�����ַ�Ӧ���ݳ�����ֱ���ü�ʯ�����գ�����b��
D. ȡa�˻����������Ba(OH)2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b�˹��塣
��1�� B��NaOH C��Na2CO3 D��NaCl E��Na2O2
��2�� 2Na2O2��2H2O===4Na����4OH����O2����CO ��H2O��CO2===2HCO
��H2O��CO2===2HCO
2NaHCO3 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��3�� C
�������������A�������ڵ�ȼʱ��������ɫ��ĩ����A��Na��E��Na2O2��A��ˮ����B����B��NaOH;NaOH��Һ��ͨ��CO2�����C��Na2CO3����Na2CO3��Һ��ͨ�������CO2�����F��NaHCO3;NaHCO3���ȶ������ȷֽ����Na2CO3��Na2CO3��HCl������Ӧ����D��NaCl����1�� A��B��C��D��E��F�Ļ�ѧʽ�ֱ���B��NaOH��C��Na2CO3��D��NaCl��E��Na2O2����2�� E��B�����ӷ���ʽ��2Na2O2��2H2O===4Na����4OH����O2����C��F�����ӷ���ʽ��CO ��H2O��CO2===2HCO
��H2O��CO2===2HCO ��F��C�Ļ�ѧ����ʽ�ǣ�2NaHCO3
��F��C�Ļ�ѧ����ʽ�ǣ�2NaHCO3  Na2CO3��H2O��CO2������3��A. ȡa�˻�����ּ��ȣ�ֻ��NaHCO3��ֽ����Na2CO3��CO2��ˮ�����ص���������CO2��ˮ�������ͣ����Ը��ݼ��������b�˾Ϳ��Լ����NaHCO3����������˾���ȷ�ⶨNa2CO3��NaHCO3�������Na2CO3�������������� B. ���߶��������ᷢ����Ӧ�����õ���������NaCl.����ԭ�������Na2CO3��NaHCO3�����ʵ����ֱ���x��y,������г������飺106x+84y=a��2x+y=b��58.5��������⣬�õ�Na2CO3������������ͻ�õ�Na2CO3�������������� C. �ڻ�������������ᷢ����Ӧʱ��������CO2��ˮ���������Ա���ʯ�����գ���˲���ȷ��CO2���������Ͳ��ܼ��������и��Ե����������Բ���ȷ��Na2CO3������������ȷ��D.���߶��ܹ���Ba(OH)2������Ӧ����BaCO3����������ԭ�������Na2CO3��NaHCO3�����ʵ����ֱ���x��y,������г������飺106x+84y=a�� x+y=b��197��������⣬���Լ���õ�Na2CO3������������ͻ�õ�Na2CO3��������������
Na2CO3��H2O��CO2������3��A. ȡa�˻�����ּ��ȣ�ֻ��NaHCO3��ֽ����Na2CO3��CO2��ˮ�����ص���������CO2��ˮ�������ͣ����Ը��ݼ��������b�˾Ϳ��Լ����NaHCO3����������˾���ȷ�ⶨNa2CO3��NaHCO3�������Na2CO3�������������� B. ���߶��������ᷢ����Ӧ�����õ���������NaCl.����ԭ�������Na2CO3��NaHCO3�����ʵ����ֱ���x��y,������г������飺106x+84y=a��2x+y=b��58.5��������⣬�õ�Na2CO3������������ͻ�õ�Na2CO3�������������� C. �ڻ�������������ᷢ����Ӧʱ��������CO2��ˮ���������Ա���ʯ�����գ���˲���ȷ��CO2���������Ͳ��ܼ��������и��Ե����������Բ���ȷ��Na2CO3������������ȷ��D.���߶��ܹ���Ba(OH)2������Ӧ����BaCO3����������ԭ�������Na2CO3��NaHCO3�����ʵ����ֱ���x��y,������г������飺106x+84y=a�� x+y=b��197��������⣬���Լ���õ�Na2CO3������������ͻ�õ�Na2CO3��������������
���㣺�������ʵ��ƶϡ�����ʽ����д��������к��еijɷֺ����IJⶨ�������жϵ�֪ʶ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д����и������У������������������Ԫ�صĵ���ֱ�ӻ��ϵõ�����
| A��FeS | B��FeCl2 | C��FeCl3 | D��Fe3O4 |
�����豸����ʱ������ѧ��ת��Ϊ���ܵ���
A����̫���ܵ�� |
B������ӵ�� |
C��̫���ܼ����� |
D��ȼ���� |
��ѧ��������������������ء������й�˵���У��������
| A�����ø����������ҩ�ý��һ�����彡�����Σ�� |
| B���Ʋ��ƻ���������ȶ�п�������ʴ |
| C���رո��ܺĵĻ�����ҵ�������ˡ���̼���á�����ּ |
| D��������Ҫ���ɻ�ʯȼ��ȼ���ŷŵĶ�������������̳��Լ�������β���������ﳾ�ȵ��µ� |