��Ŀ����
ijѧ����0.10mol/L��NaOH��Һ�ζ�ijŨ�ȵ����ᣮ��¼��������
��1���ζ�ʱ���õ�ָʾ���� ��
A��Ʒ����Һ B����̪��Һ C��ʯ����Һ
��2����ȥ��ʽ�ζ��������ݵķ���Ӧ���ò��� ��Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��
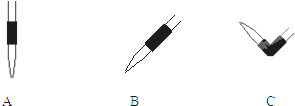
��3���ζ�����ʱ���۾�Ӧע�� ��
��4�������������ݣ�������������Ũ��ԼΪ ��������λ��Ч���֣���
��5����ƿˮϴ��δ����Բⶨ�����Ӱ���� ���ƫ�ߡ���ƫ�͡���Ӱ�족����ͬ������ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ���Բⶨ�����Ӱ���� ��
| ʵ���� | NaOH��Һ��Ũ�� ��mol/L�� | ����NaOH��Һ�� �����mL�� | ����������Һ����� ��mL�� |
| 1 | 0.10 | 19.98 | 20.00 |
| 2 | 0.10 | 20.02 | 20.00 |
| 3 | 0.10 | 20.00 | 20.00 |
A��Ʒ����Һ B����̪��Һ C��ʯ����Һ
��2����ȥ��ʽ�ζ��������ݵķ���Ӧ���ò���

��3���ζ�����ʱ���۾�Ӧע��
��4�������������ݣ�������������Ũ��ԼΪ
��5����ƿˮϴ��δ����Բⶨ�����Ӱ����
���㣺�к͵ζ�
ר�⣺ʵ����
��������1��ǿ����ǿ�Ӧ����ǿ��ǿ���Σ���Һ�����ԣ���ѡ���̪�������ָʾ����
��2�����ݼ�ʽ�ζ����������ݵķ�����
��3�������к͵ζ��У��۾�Ӧע�ӵ�����ƿ����Һ��ɫ�仯���жϵζ��յ㣻
��4���ȷ������ݵ���Ч�ԣ�������������ƽ�������Ȼ�����c���ᣩ=
���㣮
��5������c�����⣩=
����������������c�����⣩��Ӱ�죬�Դ��ж�Ũ�ȵ���
��2�����ݼ�ʽ�ζ����������ݵķ�����
��3�������к͵ζ��У��۾�Ӧע�ӵ�����ƿ����Һ��ɫ�仯���жϵζ��յ㣻
��4���ȷ������ݵ���Ч�ԣ�������������ƽ�������Ȼ�����c���ᣩ=
| c(��)��V(��) |
| V(��) |
��5������c�����⣩=
| V(��)��c(��) |
| V(����) |
���
�⣺��1��NaOH��Һ�����ᷴӦ����ǿ��ǿ���Σ���Һ�����ԣ���ѡ���̪�������ָʾ������ѡ��B��
��2����ʽ�ζ����������ݵķ������ѵζ��ܵĽ�ͷ�����������������������ݣ���ѡ��C��
��3���ζ�ʱ���۾�Ҫע������ƿ����Һ��ɫ�ı仯���ʴ�Ϊ����ƿ����Һ��ɫ�ı仯��
��4���������ݾ���Ч��ƽ������V��NaOH��Һ��=
mL=20.00mL������c���ᣩ=
=
=0.10 mol/L��
�ʴ�Ϊ��0.10 mol/L��
��5����ƿˮϴ��δ�������Һ�����ʵ������䣬V���꣩���䣬����c�����⣩=
������֪c�����⣩��Ӱ�죻��ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ�����V���꣩ƫ����c�����⣩=
������֪c�����⣩ƫ�ߣ��ʴ�Ϊ����Ӱ�죻ƫ�ߣ�
��2����ʽ�ζ����������ݵķ������ѵζ��ܵĽ�ͷ�����������������������ݣ���ѡ��C��
��3���ζ�ʱ���۾�Ҫע������ƿ����Һ��ɫ�ı仯���ʴ�Ϊ����ƿ����Һ��ɫ�ı仯��
��4���������ݾ���Ч��ƽ������V��NaOH��Һ��=
| 19.98+20.02+20.00 |
| 3 |
| c(��)��V(��) |
| V(��) |
| 0.10mol/L��20.00mL |
| 20.00mL |
�ʴ�Ϊ��0.10 mol/L��
��5����ƿˮϴ��δ�������Һ�����ʵ������䣬V���꣩���䣬����c�����⣩=
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
������������Ҫ�����˵ζ�ԭ����������ʹ�á�ָʾ����ѡ���������ȣ�����ζ��ζ�ԭ���ǽ����Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
�����Ŀ
�йؼ�ȩ��HCHO��������������̼��ˮ˵������ȷ���ǣ�������
| A��������������ԭ�ӹ�ƽ�� |
| B����ȩ�����Ͷ�����̼��̼ԭ�Ӿ�����sp2�ӻ� |
| C������������̼�ǷǼ��Է��ӣ�ˮ�ͼ�ȩ�Ǽ��Է��� |
| D��ˮ�ķе�ȼ�ȩ�ߵö࣬����Ϊˮ���Ӽ����γ����������ȩ���Ӽ䲻���γ���� |
����˵����ȷ���ǣ�������
| A����������������õ���չ�ԣ�����Ϊ���������е�ԭ�Ӳ��ڻ��������н�����δ�ƻ� |
| B��������ͼ���кڵ��������ָ�������Ķ��� |
| C�����м��Լ��ķ���һ���Ǽ��Է��� |
| D������Խ��ʾ�÷���Խ�������ȷֽ� |