��Ŀ����
��ͼ��Ϊ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�أ�

��ش��������⣺
��1����������d��Ԫ�ص��� �����ţ���
��2��д����̬��ԭ�ӵĵ����Ų�ʽ ��
��3��Ԫ�آ��γɵ�RO32-������������幹���� ��������ԭ�ӵ��ӻ���������� ��
��4��Ԫ�آٵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ���� ��
A�������к������ B�����ڷǼ��Է���
C������4���Ҽ���1���м� D�����⻯������У���ԭ�Ӳ���sp2�ӻ�
��5��Ԫ�آߣ���X��ʾ�����⻯���Ԫ�آۣ���Y��ʾ����һ���⻯�����Ҫ�������ʱȽ��£�
H2X��H2Y2��Է�������������ͬ����������������ʲ������Ҫԭ�� �� ��
��6��Ԫ�آܺ͢��γɵĻ���������������ṹ��ͼ����ʾ����û�����Ļ�ѧʽ�� �����û����ᄃ����ܶ�Ϊa g?cm-3�������ӵ�����Ϊ6.02��1023����������� ��ֻҪ���г���ʽ����

��ش��������⣺
��1����������d��Ԫ�ص���
��2��д����̬��ԭ�ӵĵ����Ų�ʽ
��3��Ԫ�آ��γɵ�RO32-������������幹����
��4��Ԫ�آٵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ����
A�������к������ B�����ڷǼ��Է���
C������4���Ҽ���1���м� D�����⻯������У���ԭ�Ӳ���sp2�ӻ�
��5��Ԫ�آߣ���X��ʾ�����⻯���Ԫ�آۣ���Y��ʾ����һ���⻯�����Ҫ�������ʱȽ��£�
| �۵�/K | �е�/K | ��״��ʱ��ˮ�е��ܽ�� | |
| H2X | 187 | 202 | 2.6 |
| H2Y2 | 272 | 423 | ������Ȼ��� |
��6��Ԫ�آܺ͢��γɵĻ���������������ṹ��ͼ����ʾ����û�����Ļ�ѧʽ��
���㣺Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ��,�жϼ��ӻ����ӵĹ���,�����ļ���,ԭ�ӹ���ӻ���ʽ���ӻ������ж�
ר�⣺Ԫ����������Ԫ�����ڱ�ר��,��ѧ���뾧��ṹ
��������Ԫ�������ڱ���λ�ã���֪��ΪC����ΪN����ΪO����ΪF����ΪMg����ΪSi����ΪS����ΪCa����ΪFe����ΪCo��
��1��d��Ԫ�ذ���3-12��Ԫ�أ���ϵԪ�ء��ϵԪ�س��⣩��
��2����ΪFe��ԭ�Ӻ�����26�����ӣ������������ԭ����д��������Ų�ʽ��
��3��Ԫ�آ��γɵ�SO32-������Sԭ�ӵŵ��Ӷ������۲���Ӷ������ݴ�ȷ���ռ乹�͡�Sԭ���ӻ���ʽ��
��4��Ԫ�آٵ�һ���⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־����û�����ΪC2H4���ṹʽΪ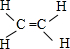 ��ƽ���ͽṹ������Ϊ�Գƽṹ�����ڷǽ����Է��ӣ�����Cԭ���γɵĦҼ���Ŀ�ж����ӻ���ʽ��
��ƽ���ͽṹ������Ϊ�Գƽṹ�����ڷǽ����Է��ӣ�����Cԭ���γɵĦҼ���Ŀ�ж����ӻ���ʽ��
��5�����������������
��6�����ݾ�̯������F��Ca������ԭ����Ŀ������ȷ����ѧʽ�����㾧��������������V=
���㾧���������
��1��d��Ԫ�ذ���3-12��Ԫ�أ���ϵԪ�ء��ϵԪ�س��⣩��
��2����ΪFe��ԭ�Ӻ�����26�����ӣ������������ԭ����д��������Ų�ʽ��
��3��Ԫ�آ��γɵ�SO32-������Sԭ�ӵŵ��Ӷ������۲���Ӷ������ݴ�ȷ���ռ乹�͡�Sԭ���ӻ���ʽ��
��4��Ԫ�آٵ�һ���⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־����û�����ΪC2H4���ṹʽΪ
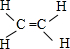 ��ƽ���ͽṹ������Ϊ�Գƽṹ�����ڷǽ����Է��ӣ�����Cԭ���γɵĦҼ���Ŀ�ж����ӻ���ʽ��
��ƽ���ͽṹ������Ϊ�Գƽṹ�����ڷǽ����Է��ӣ�����Cԭ���γɵĦҼ���Ŀ�ж����ӻ���ʽ����5������������з������
��6�����ݾ�̯������F��Ca������ԭ����Ŀ������ȷ����ѧʽ�����㾧��������������V=
| m |
| �� |
���
�⣺��Ԫ�������ڱ���λ�ã���֪��ΪC����ΪN����ΪO����ΪF����ΪMg����ΪSi����ΪS����ΪCa����ΪFe����ΪCo��
��1��d��Ԫ�ذ���3-12��Ԫ�أ���ϵԪ�ء��ϵԪ�س��⣩�����Т���d�����ʴ�Ϊ����⣻
��2����ΪFe��ԭ�Ӻ�����26�����ӣ���������Ų�ʽΪ��1S22S22P63S23P63d64S2 ���ʴ�Ϊ��1S22S22P63S23P63d64S2 ��
��3��Ԫ�آ��γɵ�SO32-��Sԭ�ӵŵ��Ӷ���=
=1���۲���Ӷ���=3+1=4����ռ乹��Ϊ�����Σ�Sԭ���ӻ���ʽΪsp3���ʴ�Ϊ�������Σ�sp3��
��4��Ԫ�آٵ�һ���⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־����û�����ΪC2H4��
A����ϩ�����в����������A����
B����ϩ����Ϊƽ��Գƽṹ��������������������غϣ����ڷǼ��Է��ӣ���B��ȷ��
C����ϩ���ӽṹʽΪ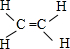 �������к���5���Ҽ���1���м�����C����
�������к���5���Ҽ���1���м�����C����
D��ÿ��C�γ�3���Ҽ���Cԭ��Ϊsp2�ӻ�����D��ȷ��
�ʴ�Ϊ��BD��
��5��H2S��H2O2��Է�������������ͬ����H2O2���Ӽ�������������ˮ���ӿ��γ�������е������ĸߣ�����ˮ������Ȼ��ܣ�
�ʴ�Ϊ��H2O2���Ӽ�������������ˮ���ӿ��γ������
��6��������Fԭ����ĿΪ8��Caԭ����Ŀ=8��
+6��
=4���ʸþ���Ļ�ѧʽΪCaF2����������=
���û����ᄃ����ܶ�Ϊa g?cm-3���������=
=
cm3��
�ʴ�Ϊ��CaF2��
cm3��
��1��d��Ԫ�ذ���3-12��Ԫ�أ���ϵԪ�ء��ϵԪ�س��⣩�����Т���d�����ʴ�Ϊ����⣻
��2����ΪFe��ԭ�Ӻ�����26�����ӣ���������Ų�ʽΪ��1S22S22P63S23P63d64S2 ���ʴ�Ϊ��1S22S22P63S23P63d64S2 ��
��3��Ԫ�آ��γɵ�SO32-��Sԭ�ӵŵ��Ӷ���=
| 6+2-2��3 |
| 2 |
��4��Ԫ�آٵ�һ���⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־����û�����ΪC2H4��
A����ϩ�����в����������A����
B����ϩ����Ϊƽ��Գƽṹ��������������������غϣ����ڷǼ��Է��ӣ���B��ȷ��
C����ϩ���ӽṹʽΪ
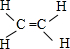 �������к���5���Ҽ���1���м�����C����
�������к���5���Ҽ���1���м�����C����D��ÿ��C�γ�3���Ҽ���Cԭ��Ϊsp2�ӻ�����D��ȷ��
�ʴ�Ϊ��BD��
��5��H2S��H2O2��Է�������������ͬ����H2O2���Ӽ�������������ˮ���ӿ��γ�������е������ĸߣ�����ˮ������Ȼ��ܣ�
�ʴ�Ϊ��H2O2���Ӽ�������������ˮ���ӿ��γ������
��6��������Fԭ����ĿΪ8��Caԭ����Ŀ=8��
| 1 |
| 8 |
| 1 |
| 3 |
| 4��78g |
| 6.02��1023 |
| ||
| ag/cm3 |
| 4��78 |
| a��6.02��1023 |
�ʴ�Ϊ��CaF2��
| 4��78 |
| a��6.02��1023 |
�����������Ƕ����ʽṹ�Ŀ��飬�漰Ԫ�����ڱ�����������Ų�ʽ�����ӽṹ���ӻ�������������������ȣ��Ƕ����ʽṹ����֪ʶ�Ŀ��飬��Ҫѧ���������ջ���֪ʶ���Ѷ��еȣ�

��ϰ��ϵ�д�
 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A���ڱ�״���£�1molˮ�������22.4L |
| B��1mol H2��ռ�����ԼΪ22.4L |
| C��3.01��1023��SO2���ӵ�����Ϊ32 |
| D���ڱ�״���£�1mol NH3��CO���������ռ�����ԼΪ22.4L |

