��Ŀ����
��ˮż����Ӧ��һ�����͵�ֱ���������Ӧ�����磺

��1����������к��еĹ����ŵ�����Ϊ ��1mol��������ȫˮ��������Ҫ���� mol NaOH��
��2����������ʹ ��Һ����дһ�֣���ɫ�����������ʽΪC10H11C1������NaOHˮ��Һ�������ɻ��������Ӧ�ķ�Ӧ����Ϊ ��
��3�����������NaOH�Ҵ���Һ�������ɻ�����������ĺ˴Ź������׳��������������壬�����֮��Ϊ1��1��1��2�����Ľṹ��ʽΪ ��
��4�����������CH3COOCH2CH3��һ����֧��ͬ���칹�壬̼�����˳ʶԳƽṹ������Cu���������O2��Ӧ�����ܷ���������Ӧ�Ļ�����������Ľṹ��ʽΪ ������������������ţ���ʹ�õ��Ⱥ�˳��д�������Լ�
��5��һ�������£� Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ ��
Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ ��

��1����������к��еĹ����ŵ�����Ϊ
��2����������ʹ
��3�����������NaOH�Ҵ���Һ�������ɻ�����������ĺ˴Ź������׳��������������壬�����֮��Ϊ1��1��1��2�����Ľṹ��ʽΪ
��4�����������CH3COOCH2CH3��һ����֧��ͬ���칹�壬̼�����˳ʶԳƽṹ������Cu���������O2��Ӧ�����ܷ���������Ӧ�Ļ�����������Ľṹ��ʽΪ
��5��һ�������£�
 Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ
Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ���㣺�л���ĺϳ�,�л�������еĹ����ż���ṹ
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
��������1���ɽṹ��ʽ��֪I�к�C=O��-COOC-��-COOC-�ɷ���ˮ�ⷴӦ��
��2��������II�к�˫�������������ʽΪC10H11C1������NaOHˮ��Һ�������ɻ������-Clת��Ϊ-OH��Ϊ±������ˮ�ⷴӦ��
��3�����ɢ��ķ���ʽΪ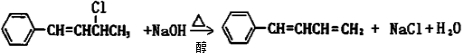 ��
��
��4�������Ǽ���-CH2OH��HOCH2-����Cu���������O2��Ӧ�����ܷ���������Ӧ��ȩ����-CHO��OHC-�����������֪V��̼�����˸���1���Ǽ���-CH2OH��HOCH2-����CH3COOCH2CH3�ķ���ʽΪC4H8O2����ȥ2��C��6��H��2��O֮��ɵ�2��C��2��H����V��̼���м�ʣ�����Ϊ-CH=CH-����V�Ľṹ��ʽΪHOCH2CH=CHCH2OH���Դ�Ϊͻ�ƿڣ������ƶ�VI�Ľṹ��ʽΪOHCCH=CHCHO��˫����-CHO����ʹ��ˮ�����������ɫ��Ӧ�ȼ���-CHO��
��5���ȽϷ�Ӧ���з�Ӧ��I��II��������Ľṹ��ʽ���ҳ�������ͬ�Ļ��źͲ���ͬ�Ļ��ţ�Ȼ���ƶϸ÷�Ӧʵ����II�������ǻ�����̼�����ѣ�I����������ͪ������̼�ϵ�̼������ѣ��ǻ�����������������ˮ�����ಿ�ֽ�������л�����ɴ���ȿɵ����Ʒ�Ӧ�ٵ��л�����Ľṹ��ʽ��
��2��������II�к�˫�������������ʽΪC10H11C1������NaOHˮ��Һ�������ɻ������-Clת��Ϊ-OH��Ϊ±������ˮ�ⷴӦ��
��3�����ɢ��ķ���ʽΪ
 ��
����4�������Ǽ���-CH2OH��HOCH2-����Cu���������O2��Ӧ�����ܷ���������Ӧ��ȩ����-CHO��OHC-�����������֪V��̼�����˸���1���Ǽ���-CH2OH��HOCH2-����CH3COOCH2CH3�ķ���ʽΪC4H8O2����ȥ2��C��6��H��2��O֮��ɵ�2��C��2��H����V��̼���м�ʣ�����Ϊ-CH=CH-����V�Ľṹ��ʽΪHOCH2CH=CHCH2OH���Դ�Ϊͻ�ƿڣ������ƶ�VI�Ľṹ��ʽΪOHCCH=CHCHO��˫����-CHO����ʹ��ˮ�����������ɫ��Ӧ�ȼ���-CHO��
��5���ȽϷ�Ӧ���з�Ӧ��I��II��������Ľṹ��ʽ���ҳ�������ͬ�Ļ��źͲ���ͬ�Ļ��ţ�Ȼ���ƶϸ÷�Ӧʵ����II�������ǻ�����̼�����ѣ�I����������ͪ������̼�ϵ�̼������ѣ��ǻ�����������������ˮ�����ಿ�ֽ�������л�����ɴ���ȿɵ����Ʒ�Ӧ�ٵ��л�����Ľṹ��ʽ��
���
�⣺��1���ɽṹ��ʽ��֪I�к�C=O��-COOC-�����Ʒֱ�Ϊ�ʻ���������-COOC-�ɷ���ˮ�ⷴӦ����1mol��������ȫˮ��������Ҫ����1molNaOH��
�ʴ�Ϊ���ʻ���������
��2��������II�к�˫������ʹ��ˮ����������Һ��ɫ�����������ʽΪC10H11C1������NaOHˮ��Һ�������ɻ������-Clת��Ϊ-OH��Ϊ±������ˮ�ⷴӦ��������ȡ����Ӧ���ʴ�Ϊ����ˮ����������Һ��ˮ�⣨��ȡ������Ӧ��
��3���������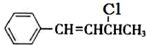 ��NaOH�Ҵ���Һ�������ɵ��л����������C6H5CH=C=CHCH3��C6H5CH=CHCH=CH2��ǰ�߲��ȶ���������IV�Ľṹ��ʽΪ
��NaOH�Ҵ���Һ�������ɵ��л����������C6H5CH=C=CHCH3��C6H5CH=CHCH=CH2��ǰ�߲��ȶ���������IV�Ľṹ��ʽΪ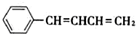 ����˴Ź������׳��������������壬�����֮��Ϊ1��1��1��2��
����˴Ź������׳��������������壬�����֮��Ϊ1��1��1��2��
�ʴ�Ϊ��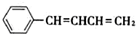 ��
��
��4�������Ǽ���-CH2OH��HOCH2-����Cu���������O2��Ӧ�����ܷ���������Ӧ��ȩ����-CHO��OHC-�����������֪V��̼�����˸���1���Ǽ���-CH2OH��HOCH2-����CH3COOCH2CH3�ķ���ʽΪC4H8O2����ȥ2��C��6��H��2��O֮��ɵ�2��C��2��H����V��̼���м�ʣ�����Ϊ-CH=CH-����V�Ľṹ��ʽΪHOCH2CH=CHCH2OH���Դ�Ϊͻ�ƿڣ������ƶ�VI�Ľṹ��ʽΪOHCCH=CHCHO�����������ʱ������������Һ����ȩ�����ټ�ϡ��������Һ�����Ժ���ˮ����̼̼˫����
�ʴ�Ϊ��OHCCH=CHCHO��������Һ��ϡ���ᡢ��ˮ��
��5���������Ϣ��֪��Ӧʵ����II�������ǻ�����̼�����ѣ�I����������ͪ������̼�ϵ�̼������ѣ��ǻ�����������������ˮ����������Ľṹ��ʽΪ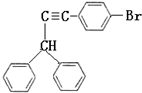 ��
��
�ʴ�Ϊ�� ��
��
�ʴ�Ϊ���ʻ���������
��2��������II�к�˫������ʹ��ˮ����������Һ��ɫ�����������ʽΪC10H11C1������NaOHˮ��Һ�������ɻ������-Clת��Ϊ-OH��Ϊ±������ˮ�ⷴӦ��������ȡ����Ӧ���ʴ�Ϊ����ˮ����������Һ��ˮ�⣨��ȡ������Ӧ��
��3���������
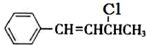 ��NaOH�Ҵ���Һ�������ɵ��л����������C6H5CH=C=CHCH3��C6H5CH=CHCH=CH2��ǰ�߲��ȶ���������IV�Ľṹ��ʽΪ
��NaOH�Ҵ���Һ�������ɵ��л����������C6H5CH=C=CHCH3��C6H5CH=CHCH=CH2��ǰ�߲��ȶ���������IV�Ľṹ��ʽΪ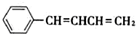 ����˴Ź������׳��������������壬�����֮��Ϊ1��1��1��2��
����˴Ź������׳��������������壬�����֮��Ϊ1��1��1��2���ʴ�Ϊ��
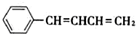 ��
����4�������Ǽ���-CH2OH��HOCH2-����Cu���������O2��Ӧ�����ܷ���������Ӧ��ȩ����-CHO��OHC-�����������֪V��̼�����˸���1���Ǽ���-CH2OH��HOCH2-����CH3COOCH2CH3�ķ���ʽΪC4H8O2����ȥ2��C��6��H��2��O֮��ɵ�2��C��2��H����V��̼���м�ʣ�����Ϊ-CH=CH-����V�Ľṹ��ʽΪHOCH2CH=CHCH2OH���Դ�Ϊͻ�ƿڣ������ƶ�VI�Ľṹ��ʽΪOHCCH=CHCHO�����������ʱ������������Һ����ȩ�����ټ�ϡ��������Һ�����Ժ���ˮ����̼̼˫����
�ʴ�Ϊ��OHCCH=CHCHO��������Һ��ϡ���ᡢ��ˮ��
��5���������Ϣ��֪��Ӧʵ����II�������ǻ�����̼�����ѣ�I����������ͪ������̼�ϵ�̼������ѣ��ǻ�����������������ˮ����������Ľṹ��ʽΪ
 ��
���ʴ�Ϊ��
 ��
��
���������⿼���л���ĺϳɣ�Ϊ��Ƶ���㣬�����л�����ʹ��������ɺ������Լ��������ϵ����Ҫ�л���Ӧ������ȡ����Ӧ���ӳɷ�Ӧ����ȥ��Ӧ��������Ӧ���˴Ź������������ͷ������Ϊ���Ĺؼ����ۺϿ��鿼���ķ����������������������ۺ�Ӧ����Ϣ�Լ���ϢǨ��������

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
�����Ŀ
 800��ʱ�����ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO��H2Oά�ֺ��£�������ӦCO��g��+H2O��g��?H2��g��+CO2��g������Ӧ�����вⶨ�IJ������ݼ��±���
800��ʱ�����ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO��H2Oά�ֺ��£�������ӦCO��g��+H2O��g��?H2��g��+CO2��g������Ӧ�����вⶨ�IJ������ݼ��±��� ��һ��������þ���Ͻ�Ͷ��100mLһ��Ũ�ȵ������У��Ͻ���ȫ�ܽ⣮��������Һ�еμ�Ũ��Ϊ5mol/L��NaOH��Һ�����ɵij����������NaOH��Һ�������ϵ��ͼ���������������λ��mL��������������λ��g����
��һ��������þ���Ͻ�Ͷ��100mLһ��Ũ�ȵ������У��Ͻ���ȫ�ܽ⣮��������Һ�еμ�Ũ��Ϊ5mol/L��NaOH��Һ�����ɵij����������NaOH��Һ�������ϵ��ͼ���������������λ��mL��������������λ��g���� �����Ľṹ��ͼ��ʾ�����ɿ��������ĸ���ͬ����Ԫ����ɵĿռ乹�ͣ�
�����Ľṹ��ͼ��ʾ�����ɿ��������ĸ���ͬ����Ԫ����ɵĿռ乹�ͣ�

