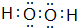��Ŀ����
�±���Ԫ�����ڱ���ǰ�����ڣ��ش��������⣺

��1��f��gԪ�صĵ��ʹ�5.0g��100mLˮ����ȫ��Ӧ�����ɵ���Һ��ֻ����һ�����ʣ��ֲ�д��������Ӧ�Ļ�ѧ����ʽ����
��2��a��b��c��d��e���⻯��ķе�ֱ������ͼ��ͼ��ʾ����š�5���⻯��Ļ�ѧʽΪ
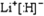 ��
��

��1��f��gԪ�صĵ��ʹ�5.0g��100mLˮ����ȫ��Ӧ�����ɵ���Һ��ֻ����һ�����ʣ��ֲ�д��������Ӧ�Ļ�ѧ����ʽ����
2Na+2H2O=2NaOH+H2��
2Na+2H2O=2NaOH+H2��
��2Al+2NaOH+2H2O=2NaAlO2+3H2��
2Al+2NaOH+2H2O=2NaAlO2+3H2��
��������Һ�����ʵ���Ũ�����ֵΪ1mol/L
1mol/L
����Һ�������仯���Բ��ƣ���2��a��b��c��d��e���⻯��ķе�ֱ������ͼ��ͼ��ʾ����š�5���⻯��Ļ�ѧʽΪ
CH4
CH4
����š�1���⻯��ĵ���ʽΪ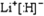
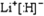

��������1��fΪNa��gΪAl����100mLˮ����ȫ��Ӧ�����ɵ���Һ��ֻ����һ�����ʣ�������ΪNaAlO2����Al��NaOHǡ����ȫ��Ӧ��
��2���ɷе��֪����š�5���⻯����ͣ���š�1���⻯������a��b��c��d��e���⻯���У�LiHΪ���ӻ�����е����b��c��d��e�ķе�������b���⻯�����ķе���ͣ�
��2���ɷе��֪����š�5���⻯����ͣ���š�1���⻯������a��b��c��d��e���⻯���У�LiHΪ���ӻ�����е����b��c��d��e�ķе�������b���⻯�����ķе���ͣ�
����⣺��1��fΪNa��gΪAl����100mLˮ����ȫ��Ӧ�����ɵ���Һ��ֻ����һ�����ʣ�������ΪNaAlO2����Al��NaOHǡ����ȫ��Ӧ�������ķ�Ӧ��2Na+2H2O=2NaOH+H2����
2Al+2NaOH+2H2O=2NaAlO2+3H2����5g������Na��Al�����ʵ�����ͬ����Ϊ0.1mol������0.1molNaAlO2����Һ�������Ϊ100mL����c=
=1mol/L��
�ʴ�Ϊ��2Na+2H2O=2NaOH+H2����2Al+2NaOH+2H2O=2NaAlO2+3H2����1mol/L��
��2���ɷе��֪����š�5���⻯����ͣ���š�1���⻯������a��b��c��d��e���⻯���У�LiHΪ���ӻ�����е����c��d��e���⻯�����к����������b��c��d��e�ķе�������b���⻯�����ķе���ͣ�������š�5���⻯��Ļ�ѧʽΪCH4����š�1���⻯��ĵ���ʽΪ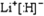 ��
��
�ʴ�Ϊ��CH4��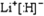 ��
��
2Al+2NaOH+2H2O=2NaAlO2+3H2����5g������Na��Al�����ʵ�����ͬ����Ϊ0.1mol������0.1molNaAlO2����Һ�������Ϊ100mL����c=
| 0.1mol |
| 0.1L |
�ʴ�Ϊ��2Na+2H2O=2NaOH+H2����2Al+2NaOH+2H2O=2NaAlO2+3H2����1mol/L��
��2���ɷе��֪����š�5���⻯����ͣ���š�1���⻯������a��b��c��d��e���⻯���У�LiHΪ���ӻ�����е����c��d��e���⻯�����к����������b��c��d��e�ķе�������b���⻯�����ķе���ͣ�������š�5���⻯��Ļ�ѧʽΪCH4����š�1���⻯��ĵ���ʽΪ
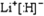 ��
���ʴ�Ϊ��CH4��
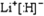 ��
�����������⿼��λ�á��ṹ�����ʣ��������ʵ����ʵĿ��飬ע��Al��NaOH�ķ�Ӧ���е�Ƚϼ��ɽ����ȷ�е�Ϊ���Ӿ�����ڷ��Ӿ��壬�Һ���������ʷе�ߣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д�
�����Ŀ
 �±���Ԫ�����ڱ���ǰ�����ڣ�
�±���Ԫ�����ڱ���ǰ�����ڣ� �±���Ԫ�����ڱ���ǰ�����ڣ�
�±���Ԫ�����ڱ���ǰ�����ڣ�