��Ŀ����
12��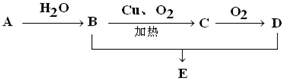 ��ͼ��ʾ����֪A�IJ�����һ������ʯ�ͻ���ˮƽ�ı�־��D�������ԣ�E�Ǿ�����ζ�IJ�����ˮ��Һ�壮
��ͼ��ʾ����֪A�IJ�����һ������ʯ�ͻ���ˮƽ�ı�־��D�������ԣ�E�Ǿ�����ζ�IJ�����ˮ��Һ�壮��1��A��������ϩ����ṹ��ʽΪCH2=CH2��
��2��A��B�Ļ�ѧ��Ӧ����ʽCH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH�����ڼӳɷ�Ӧ��
��3��д��C��D�Ļ�ѧ��Ӧ����ʽ2CH3CHO+O2$\stackrel{����}{��}$2CH3COOH+2H2O������������Ӧ��
���� A�IJ�����һ������ʯ�ͻ���ˮƽ�ı�־����A��CH2=CH2����ϩ��ˮ�����ӳɷ�Ӧ����B��CH3CH2OH����ͭ�����������������£��Ҵ�����������CΪCH3CHO����ȩ����������D��D�������ԣ���D��CH3COOH����Ũ�����������������������Ҵ������ᷢ��������Ӧ����E��E�Ǿ�����ζ�IJ�����ˮ��Һ�壬E��CH3COOCH2CH3��
��� �⣺A�IJ�����һ������ʯ�ͻ���ˮƽ�ı�־����A��CH2=CH2����ϩ��ˮ�����ӳɷ�Ӧ����B��CH3CH2OH����ͭ�����������������£��Ҵ�����������CΪCH3CHO����ȩ����������D��D�������ԣ���D��CH3COOH����Ũ�����������������������Ҵ������ᷢ��������Ӧ����E��E�Ǿ�����ζ�IJ�����ˮ��Һ�壬E��CH3COOCH2CH3��
��1��ͨ�����Ϸ���֪��A����ϩ���ṹ��ʽΪCH2=CH2��
�ʴ�Ϊ����ϩ��CH2=CH2��
��2��A��B����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����Ӧ����ʽΪ��CH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH���÷�Ӧ���ڼӳɷ�Ӧ��
�ʴ�Ϊ��CH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH���ӳɣ�
��3��C��D����ȩ�������������ᣬ��Ӧ����ʽΪ��2CH3CHO+O2$\stackrel{����}{��}$2CH3COOH+2H2O���÷�Ӧ����������Ӧ��
�ʴ�Ϊ��2CH3CHO+O2$\stackrel{����}{��}$2CH3COOH+2H2O��������
���� ���⿼���л�����ƶϣ��Ƚϻ������漰ϩ��������ȩ�����ᡢ����������ת����ע�����֪ʶ�����գ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| A�� | ��Na2SiO3��Һ�е����̪��Һ���������� | |
| B�� | �Թܢ��з�Ӧ�����ӷ���ʽ�ǣ�Na2CO3+2H+�T2Na++CO2��+H2O | |
| C�� | ��ʵ�����֤�����ԣ����̼����� | |
| D�� | �������ỻ�����ᣬ��֤�����ԣ����̼����� |
| A�� | NaCl�������ӻ����� | B�� | ԭ����ΪOH-�����ǻ� | ||
| C�� | �Ҵ���ˮ��Ϻ��ã������ֲַ� | D�� | ����ˮ������ղ��ﺬ�������� |

| A�� | ����ʯī���������������� | |
| B�� | �����ĵ缫��Ӧʽ��Cl-+2OH--2e-=ClO-+H2O | |
| C�� | �����������������������Һ�е������������缫�˶� | |
| D�� | ��ȥCN-�ķ�Ӧ��2CN-+5ClO-+2H+=N2��+2CO2��+5Cl-+H2O |
 �ȼҵ�Ե�⾫�Ʊ���ʳ��ˮ�ķ�����ȡ�������������ռ���ȵĺ������ε�ϵ�л�����Ʒ����ͼ�����ӽ���Ĥ�����ʳ��ˮ��ʾ��ͼ��ͼ�е����ӽ���Ĥֻ����������ͨ����
�ȼҵ�Ե�⾫�Ʊ���ʳ��ˮ�ķ�����ȡ�������������ռ���ȵĺ������ε�ϵ�л�����Ʒ����ͼ�����ӽ���Ĥ�����ʳ��ˮ��ʾ��ͼ��ͼ�е����ӽ���Ĥֻ����������ͨ�������������գ�
��1��д����ⱥ��ʳ��ˮ�����ӷ���ʽ2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2OH-+H2��+Cl2����
��2�����Ʊ���ʳ��ˮ��ͼ��aλ�ò��䣬����������Һ��ͼ��dλ����������ѡ�a������b������c����d����
��3��KClO3���ԺͲ��ᣨH2C2O4�������ᷴӦ���ɸ�Ч������ɱ����ClO2��������CO2��KHSO4�����ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ2KClO3+H2C2O4+2H2SO4=2ClO2+2CO2+2KHSO4+2H2O��
��4����֪�����¼��������ƽ�ⳣ����
| K1 | K2 | |
| H2SO3 | 1.54x 10-2 | 1.02x 10-7 |
| HClO | 2.95x 10-8 | |
| H2CO3 | 4.3x 10-7 | 5.6x 10-11 |
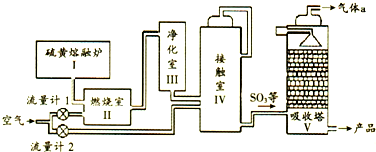
| A�� | ������Ҫ��Ӧ���Ȼ�ѧ����ʽ��S��s��+O2��g��?SO3��g����H=-297kJ•mol-1S��s��+O2��s��=SO2��g����H=-297kJ•mol-1 | |
| B�� | ������Ҫ��Ӧ�Ļ�ѧ����ʽ��2SO2+O2��s�� $?_{��}^{����}$SO3 | |
| C�� | ����ʹ�ô��������ѧ��Ӧ���ʺ�ƽ��ת���� | |
| D�� | ��������a ����ֱ���ŷŵ������� |
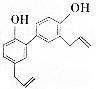 ��ҩ����Ƥ�������õ���Ч�ɷ�Ϊ���ӷӣ���ṹ��ʽ��ͼ��ʾ�������ʾ��г־õļ����ɳ����úͼ�ǿ�Ŀ������ã��ٴ�����Ҫ������������������ȣ�
��ҩ����Ƥ�������õ���Ч�ɷ�Ϊ���ӷӣ���ṹ��ʽ��ͼ��ʾ�������ʾ��г־õļ����ɳ����úͼ�ǿ�Ŀ������ã��ٴ�����Ҫ������������������ȣ������йظ����ʵ�������ȷ���ǣ�������
| A�� | 1�����ӷӷ����к���16��̼ԭ�� | |
| B�� | ���ӷ���ʹ����KMnO4��Һ��ɫ����FeCl3��Һ��ɫ | |
| C�� | 1mol���ӷ�������NaHCO3��Һ��Ӧ�ų�22.4LCO2����״���� | |
| D�� | 1mol���ӷ�����4molBr2��Br2��CCl4��Һ����Ӧ |



 ��
�� ������һ�֣�����дһ�֣���
������һ�֣�����дһ�֣��� $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ +H2O��
+H2O��