��Ŀ����
10���л���AΪ����������ͼ��������Է�������Ϊ70������ط�Ӧ��ͼ��ʾ������B��D��E�Ľṹ�о�����2��-CH3�����ǵĺ˴Ź��������о�����4���壮
��ش�
��1��B�����������ŵ�����Ϊ��ԭ�ӣ�D�ķ���ʽΪC5H10O��
��2����ķ�Ӧ����Ϊab������ĸ��ţ���
a����ԭ��Ӧ b���ӳɷ�Ӧ c��������Ӧ d����ȥ��Ӧ
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��
 +NaOH$��_{��}^{�Ҵ�}$
+NaOH$��_{��}^{�Ҵ�}$ +NaBr+H2O��
+NaBr+H2O����
 +HBr$\stackrel{��}{��}$
+HBr$\stackrel{��}{��}$ +H2O��
+H2O����4��C��E����һ�������·�Ӧ����F��FΪ����ζ���л�������÷�Ӧ�Ļ�ѧ����ʽΪ
 ��
����5��A��ͬ���칹������һ�Ի�Ϊ˳���칹���ҽṹ����2��-CH3�����ǵĽṹ��ʽΪ
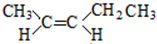 ��
��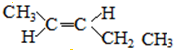 ��
����6��E����һ��ͬ���칹���ܷ���������Ӧ�����������������������������ܷ�����ȥ��Ӧ����ṹ��ʽΪ
 ��
��
���� �л���AΪ����������ͼ��������Է�������Ϊ70��������Cԭ�������ĿΪ$\frac{70}{12}$=5��10����A�ķ���ʽΪC5H10��Bת��ΪA������ȥ��Ӧ����AΪϩ������ת����ϵ��֪��DΪȩ��EΪ���ᡢCΪ����BΪ±�������Ҿ�Ϊ�������ţ�����B��D��E�Ľṹ�о�����2��-CH3�����ǵĺ˴Ź��������о�����4���壬��DΪ��CH3��2CHCH2CHO��CΪ��CH3��2CHCH2CH2OH��BΪ��CH3��2CHCH2CH2Br��AΪ��CH3��2CHCH=CH2��EΪ��CH3��2CHCH2COOH���ݴ˽��
��� �⣺�л���AΪ����������ͼ��������Է�������Ϊ70��������Cԭ�������ĿΪ$\frac{70}{12}$=5��10����A�ķ���ʽΪC5H10��Bת��ΪA������ȥ��Ӧ����AΪϩ������ת����ϵ��֪��DΪȩ��EΪ���ᡢCΪ����BΪ±�������Ҿ�Ϊ�������ţ�����B��D��E�Ľṹ�о�����2��-CH3�����ǵĺ˴Ź��������о�����4���壬��DΪ��CH3��2CHCH2CHO��CΪ��CH3��2CHCH2CH2OH��BΪ��CH3��2CHCH2CH2Br��AΪ��CH3��2CHCH=CH2��EΪ��CH3��2CHCH2COOH��
��1��BΪ��CH3��2CHCH2CH2Br�����������ŵ�����Ϊ��ԭ�ӣ�DΪ��CH3��2CHCH2CHO������ʽΪC5H10O��
�ʴ�Ϊ����ԭ�ӣ�C5H10O��
��2����Ӧ���ǣ�CH3��2CHCH2CHO�����������ӳɷ�Ӧ���ɣ�CH3��2CHCH2CH2OH��Ҳ���ڻ�ԭ��Ӧ��
�ʴ�Ϊ��ab��
��3����Ӧ��ķ���ʽΪ +NaOH$��_{��}^{�Ҵ�}$
+NaOH$��_{��}^{�Ҵ�}$ +NaBr+H2O����Ӧ��ķ���ʽΪ
+NaBr+H2O����Ӧ��ķ���ʽΪ +HBr$\stackrel{��}{��}$
+HBr$\stackrel{��}{��}$ +H2O��
+H2O��
�ʴ�Ϊ�� +NaOH$��_{��}^{�Ҵ�}$
+NaOH$��_{��}^{�Ҵ�}$ +NaBr+H2O��
+NaBr+H2O�� +HBr$\stackrel{��}{��}$
+HBr$\stackrel{��}{��}$ +H2O��
+H2O��
��4��C��E����һ�������·�Ӧ����F��FΪ����ζ���л����������������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��5����CH3��2CHCH=CH2��ͬ���칹������һ�Ի�Ϊ˳���칹���ҽṹ����2��-CH3�����ǵĽṹ��ʽΪ��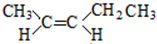 ��
��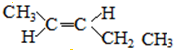 ��
��
�ʴ�Ϊ��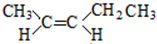 ��
��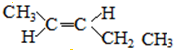 ��
��
��6����CH3��2CHCH2COOH����һ��ͬ���칹���ܷ���������Ӧ������������������������������-CHO��-OH���Ҳ��ܷ�����ȥ��Ӧ����ṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϣ��漰±����������ȩ�������������ת��������ȷ��A�ķ���ʽ�ǹؼ���ע���������չ����ŵ�������ת�����Ѷ��еȣ�

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�| A�� | ��״���µ�22.4L������ȫȼ�գ����ɶ�����̼������Ϊ8NA | |
| B�� | 50mL12mol•L-1����������MnO2���ȣ�ת�Ƶĵ�����Ϊ0.3NA | |
| C�� | ��ͬ������N2��CO�����еķ�������ԭ��������������������� | |
| D�� | 1 mol CH5+�к��еĵ�����ĿΪ11NA |
| A�� | ���Σ���SO42-��Ca2+�ȣ��ᴿֻ�漰�����仯 | |
| B�� | ��ⱥ��ʳ��ˮ���Ƶý����� | |
| C�� | ��ˮ�����漰��������ԭ��Ӧ | |
| D�� | �������ֻ�漰�����仯 |
| A�� | ��6molCuFeS2����6molCu��������16.5molO2 | |
| B�� | ��ұ�������в����������к�����SO2���ɻ���������ȡ���� | |
| C�� | �ڷ�Ӧ���У�Cu2S���������������ǻ�ԭ�� | |
| D�� | �ڷ�Ӧ���У�SO2��������������ǻ�ԭ���� |
| A�� | 46g NO2��N2O4�Ļ�������к��е�ԭ�Ӹ���Ϊ3NA | |
| B�� | �����£�4 g CH4����NA��C-H���ۼ� | |
| C�� | 10 mL��������Ϊ98%��H2SO4����ˮ��100 mL��H2SO4����������Ϊ9.8% | |
| D�� | 25��ʱ��pH=12��1.0 LNaClO��Һ��ˮ�������OH-����ĿΪ0.01NA |
| A�� | HCl��HBr��HI���ۡ��е�������������Ӽ���������С�й� | |
| B�� | H2O���ۡ��е����H2S������H2O����֮�������� | |
| C�� | I2������CCl4��������������ԭ������ | |
| D�� | �������ˮ�γ�������ֻ�ѧ�� |

��֪���ٲ��ֽ����������↑ʼ��������ȫ����ʱ��pH��
| �������� | Fe��OH��3 | Al��OH��3 | Mg��OH��2 |
| ��ʼ����pH | 2.7 | 3.7 | 9.6 |
| ��ȫ����pH | 3.7 | 4.7 | 11 |
| �¶�/�� | 0 | 10 | 20 | 50 | 75 | 100 |
| Li2CO3���ܽ��/g | 1.539 | 1.406 | 1.329 | 1.181 | 0.866 | 0.728 |
��1������������ʽ��ʾLiAlSi2O6����ɣ�Li2O•Al2O3•4SiO2��
��2����Ӧ�����̼��Ƶ������dz�ȥ��Ӧ���й�����H2SO4������pH��ʹFe3+��Al3+��ȫ������
��3��д����Ӧ�������ɳ���A�����ӷ���ʽ��Mg2++2OH-�TMg��OH��2����Ca2++CO32-�TCaCO3����
��4����Ӧ������Li2CO3������д����ʵ�����еõ�Li2CO3�����IJ������ƹ��ˣ�ϴ������Li2CO3����Ҫʹ����ˮ ��ѡ���ˮ������ˮ��������ѡ���������Li2CO3�ڽϸ��¶����ܽ��С������ˮϴ�ӿɼ���Li2CO3����ģ�
��5����������Ȼ�������ʱ�����������������л��������������ԭ���ǣ���������LiCl��Һʱ��LiCl������ˮ������LiOH�����ȷֽ�����Li2O�����ʱ����O2��
 ��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺
��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺ ��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3��
��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3�� ��
��