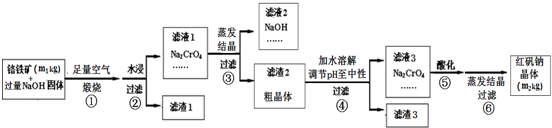
��Ŀ����
 �Ѱۣ���Ҫ�ɷ���Ti02������������ǿ�������ʸߣ������㷺�������ᡢ���ϡ���ֽ����ҵ�����������Ҵ���ˮ������Ĵ�������ͼ������������Ҫ�ɷ�FeTi03������������Ϊ��Ҫԭ�������Ѱ۵Ĺ�������ͼ���ش��������⣺
�Ѱۣ���Ҫ�ɷ���Ti02������������ǿ�������ʸߣ������㷺�������ᡢ���ϡ���ֽ����ҵ�����������Ҵ���ˮ������Ĵ�������ͼ������������Ҫ�ɷ�FeTi03������������Ϊ��Ҫԭ�������Ѱ۵Ĺ�������ͼ���ش��������⣺��1���������������м�������A��Ŀ���Ƿ�ֹFe2+������������A��
��2��д��TiOS04��Һ�ڼ���������ˮ�ⷴӦ�����ӷ���ʽ��
��3��Ϊ�ⶨTiOS04�ĺ���������ȡ������Һ10mL��ˮϡ����100mL���ӹ������ۣ������ʹ����ȫ��Ӧ��3Ti02++Al+6H+�T3Ti3++AP++3H20�����˺�ȡ����Һ20mL�������еμ�2��3��KSCN��Һ��ָʾ��������ʽ�ζ��ܵμ�0.1mol?L-1 FeCl3��Һ������Һ���ֺ�ɫ�ﵽ�ζ��յ㣬��ȥ��30mL FeC13��Һ��������Һ��TiOS04�����ʵ���Ũ����
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��
ר�⣺ʵ�������
��������������Ҫ�ɷ�FeTi03����������������80%����ֽ⣬����FeSO4��TiOS04��Һ��Ϊ��ֹTiOS04��Һ��FeSO4���������뻹ԭ���������ۣ�ͨ���ᾧ�����˵õ�FeSO4?7H2O���壬����Һ��TiOS0��ˮ������H2TiO3�������������ˡ���ɵõ��Ѱۣ�
��1����ֹFe2+�������������ۣ������Ʊ�Ti02�Ĺ����У����Եõ��ĸ������������������壬������Ի������ã�
��2��ǿ��������ˮ��õ�ǿ������
��3�����ù�ϵʽTi02+----Ti3+-----Fe3+
1 1 1
���TiOS04�����ʵ���Ũ���ǣ�
��1����ֹFe2+�������������ۣ������Ʊ�Ti02�Ĺ����У����Եõ��ĸ������������������壬������Ի������ã�
��2��ǿ��������ˮ��õ�ǿ������
��3�����ù�ϵʽTi02+----Ti3+-----Fe3+
1 1 1
���TiOS04�����ʵ���Ũ���ǣ�
���
�⣺��������Ҫ�ɷ�FeTi03����������������80%����ֽ⣬����FeSO4��TiOS04��Һ��Ϊ��ֹTiOS04��Һ��FeSO4���������뻹ԭ���������ۣ�ͨ���ᾧ�����˵õ�FeSO4?7H2O���壬����Һ��TiOS0��ˮ������H2TiO3�������������ˡ���ɵõ��Ѱۣ�
��1����Fe2+�ױ�������������Fe3+��Ϊ��ֹFe2+�����������������A�����ۣ��䷴Ӧԭ��Ϊ2Fe3++Fe=3Fe2+��ͬʱ���������µ����ʣ��Ʊ�Ti02�Ĺ����У����õ��ĸ�������FeSO4?7H2O���ɻ������õ�������H2SO4���ʴ�Ϊ�����ۣ�FeSO4?7H2O��H2SO4��
��2��TiOS04��Һ�ڼ���������ˮ�ⷴӦ�����ӷ���ʽ��������ͼ��֪��Ti��H2TiO3��������ʽ���֣�������ʽΪ��TiO2++2H2O=H2TiO3��+2H+��
�ʴ�Ϊ��TiO2++2H2O=H2TiO3��+2H+��
��3����Ӧ�����ӷ���ʽΪ��Fe3++Ti3+=Fe2++Ti4+��Fe3+��Ti3+��1��1����ʽ��Ӧ��n��Fe3+��=30��10-3��0.1=0.003mol����20 mL��Һ��n��Ti4+��=0.003mol����TiԪ���غ��֪n��TiOS04��=0.003mol����100 mL��Һ��n��Ti4+��=0.015mol��������Һ��TiOS04�����ʵ���Ũ����c��TiOS04��=
=1.5mol?L-1��
�ʴ�Ϊ��1.5mol?L-1��
��1����Fe2+�ױ�������������Fe3+��Ϊ��ֹFe2+�����������������A�����ۣ��䷴Ӧԭ��Ϊ2Fe3++Fe=3Fe2+��ͬʱ���������µ����ʣ��Ʊ�Ti02�Ĺ����У����õ��ĸ�������FeSO4?7H2O���ɻ������õ�������H2SO4���ʴ�Ϊ�����ۣ�FeSO4?7H2O��H2SO4��
��2��TiOS04��Һ�ڼ���������ˮ�ⷴӦ�����ӷ���ʽ��������ͼ��֪��Ti��H2TiO3��������ʽ���֣�������ʽΪ��TiO2++2H2O=H2TiO3��+2H+��
�ʴ�Ϊ��TiO2++2H2O=H2TiO3��+2H+��
��3����Ӧ�����ӷ���ʽΪ��Fe3++Ti3+=Fe2++Ti4+��Fe3+��Ti3+��1��1����ʽ��Ӧ��n��Fe3+��=30��10-3��0.1=0.003mol����20 mL��Һ��n��Ti4+��=0.003mol����TiԪ���غ��֪n��TiOS04��=0.003mol����100 mL��Һ��n��Ti4+��=0.015mol��������Һ��TiOS04�����ʵ���Ũ����c��TiOS04��=
| 0.015 |
| 0.01 |
�ʴ�Ϊ��1.5mol?L-1��
�����������������Ʊ����������⣬�ǽ���߿����ȵ㣬��Ҫ�����˵��ԭ����Ӧ�á����ʵ���Ũ�ȵ��йؼ��㡢����ˮ���֪ʶ�㣬�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��ijԭ�ӵ�Ħ��������M g?mol-1�������ӵ�����ΪNA mol-1��һ��12C������Ϊm g����һ����ԭ�ӵ���ʵ�����ǣ�������
A��
| ||
B��
| ||
| C��M?NA g | ||
D��
|
�����������ڴ�������ǣ�������
| A��ϡ���� | B��NaOH |
| C��Һ��ʯ���� | D����Ȼ�� |
�Ѻ������������й�ÿ��Ҫ����5�ڶ����ҵ�����ʯ��ռ���纣������ʯó������һ�����ϣ�����ȫ������ʯ�۸�����ǣ��й�������ҵЭ����Ĵ����DZغͱ��ع�˾̸��������������������ʯ˵����ȷ���ǣ�������
| A�����������Ҫ�ɷ���Fe3O4 |
| B������ʯ����Ҫ�ɷ����������Ҫ�ɷ���ͬ |
| C���������ĩ�����������KSCN��Һ����Һ���ɫ |
| D��FeO�׳����� |
���ڿ��淴Ӧ2SO2��g��+O2��g��?2SO3��g����H��0�������о�Ŀ�ĺ�ͼʾ������ǣ�������
| A | B | C | D | |
| �о�Ŀ�� | ѹǿ�Է�Ӧ��Ӱ�� | �¶ȶԷ�Ӧ��Ӱ�� | ����O2Ũ�ȶԷ�Ӧ��Ӱ�� | Ũ�ȶ�ƽ�ⳣ����Ӱ�� |
| ͼʾ | 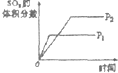 |  | 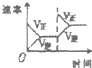 |  |
| A��A | B��B | C��C | D��D |
����ʵ�������ȷ���ǣ�������
| A����pH��ֽ���ij������ˮ��pHֵΪ3.5 |
| B����ⷨ����ͭ������ͭ�ӵ�Դ������ |
| C�����Ȼ���������������ˮ�У������Ȼ�����Һ |
| D�����Ȼ�����Һ�������ɣ������գ��͵õ��Ȼ������� |